Article Text
Abstract
OBJECTIVE To investigate changes over time in the prevalence at live birth of cardiovascular malformations and to compare “anatomical” and “physiological” diagnostic hierarchies within a population.
DESIGN Retrospective and prospective ascertainment of all congenital cardiovascular malformations diagnosed in infancy.
SETTING The resident population of one health region.
PATIENTS All infants live born from 1985 to 1997 with cardiovascular malformations confirmed by echocardiography, cardiac catheterisation, surgery or autopsy.
MAIN OUTCOME MEASURES Year to year variation in prevalence of individual malformations and of “complex”, “significant”, and “minor” groups.
RESULTS 2671 babies with cardiovascular malformations were confirmed in a denominator population of 477 960 live births (5.6 per 1000). There was no change over 13 years in the birth prevalence of “complex” or “significant” defects, but a highly significant increase in “minor” defects (p < 0.0001), mainly small ventricular septal defects. Termination of pregnancy increased from no cases in 1985 to 16 in 1997 with no demonstrable effect on live born babies with heart defects. A one dimensional “anatomical” diagnostic hierarchy led to under ascertainment of pulmonary atresia by 27%, coarctation of the aorta by 39%, and interruption of the aorta by 100%.
CONCLUSIONS The apparent increase in live born cardiovascular malformations results mainly from improved diagnosis of minor defects. There has been no change over time in birth prevalence of more serious defects. Spontaneous year to year variation in numbers will make it difficult to ascribe any short term changes to any particular intervention. A two dimensional diagnostic hierarchy is offered as a standard.
- congenital heart defects
- epidemiology
- infancy
- temporal variability
Statistics from Altmetric.com
Cardiovascular malformations account for about 10% of all infant deaths and nearly half of all deaths from malformation.1 As a group they are the most common type of congenital malformation.2 The cause of an increasing number of cardiovascular malformations is known to be genetic and a few are environmental in origin, but for the majority the cause is unknown. The descriptive epidemiology of cardiovascular malformations has generated much interest over the last 30–40 years but has not so far provided major clues to aetiology. There have been many descriptions of disease frequency but comparison between them is hampered by the lack of a common methodology. Early studies are limited by their inclusion of many unconfirmed clinical diagnoses (before ultrasound was widely available)3 and some more recent studies, which are institution based, are limited by their inability to define the population from which their patients were derived.4 Comparisons between studies are also made difficult by uncertainties over ascertainment and because of the different diagnostic categories and diagnostic hierarchies employed.5
The pattern of cardiovascular malformation is likely to be changing with time. Recognition and description of this has important implications for the future provision of services but may also give clues to the cause. Increasing antenatal recognition of congenital heart disease and termination of affected pregnancies might be expected to reduce the prevalence at live birth of the more severe abnormalities.6 Changes over time may also reflect the random effect of small numbers5 or the increasing ascertainment of minor malformations with better non-invasive technology.7 ,8 The recognised increased risk to offspring of survivors of congenital heart disease might be expected, all other things being equal, to lead to a significant increase in the birth prevalence in the population over several generations.9
Our study aimed to: (1) define the birth prevalence of cardiovascular malformations in a well defined population in the era of readily accessible non-invasive imaging; (2) propose an acceptable diagnostic hierarchy to enable comparison with previous and future reports; and (3) to examine temporal variability in the birth prevalence of cardiovascular malformations.
Methods
POPULATION
The former Northern Health Region comprises the counties of Cumbria, Northumberland, Tyne and Wear, Durham, and Cleveland, and has a population of just over three million people. Babies with suspected heart disease from the health district of South Cumbria are referred elsewhere for geographical reasons. All babies with suspected heart disease from the other 15 of 16 health districts are referred to a single paediatric cardiology centre.10
DATA SOURCES
All cases of live born cardiovascular malformation diagnosed in the first 12 months of life were identified from the diagnostic database in the regional paediatric cardiology unit in the Freeman Hospital, Newcastle upon Tyne, and were cross checked with the northern congenital abnormality survey.11 The paediatric cardiology database was established in 1990 with prospective registration of all congenital heart defects since then. Ascertainment of cases born in 1985 to 1989 was retrospective from ward admission lists, hospital diagnostic coding, and the northern regional perinatal, late neonatal, and infant mortality survey, and is thought to be complete for all complex and significant malformations (see below). There is likely to be some under ascertainment of minor malformations in the early part of the study. The perinatal, late neonatal, and infant mortality survey also provided information on all terminations of pregnancy where a heart defect had been recognised antenatally and all cardiovascular malformations in live born babies where death occurred before diagnosis had been made.12 The Office of National Statistics provided data on the regional birth rate.
CASE DEFINITION
In this study, as in most previous studies, cardiovascular malformations were defined as by Mitchell and colleagues13—that is, “a gross structural abnormality of the heart or intrathoracic great vessels that is actually or potentially of functional importance”. To enable comparison with previous studies, cases of isolated cardiac arrhythmia, cardiomyopathy, acquired heart disease, isolated bicuspid aortic valve, mitral valve prolapse without regurgitation, isolated dextrocardia, cardiac tumours, patent ductus arteriosus associated with prematurity (that is, ductus requiring surgical ligation within six weeks of the due date of delivery), atrial septal defect undergoing spontaneous closure in infancy, and mild physiological pulmonary artery branch stenosis were excluded. Ascertainment was limited to malformations diagnosed by the age of 12 months. While most significant heart disease has presented by this time, relatively few cases of patent ductus and atrial septal defect are diagnosed by 12 months of age and even fewer have undergone treatment by this age.10 Because of this, data on isolated atrial septal defect and isolated patent ductus arteriosus diagnosed in infancy are presented separately.
DIAGNOSTIC HIERARCHY
Analysis of babies with multiple cardiovascular malformations is a difficult problem and all previous reports have allocated all cases a single diagnosis based on the malformation judged most important. There has been no consensus on an acceptable diagnostic hierarchy. Many previous studies have adopted either the classification proposed in the New England regional infant cardiac programme which defines a hierarchy based mainly on anatomical severity14 or that in the Baltimore-Washington infant study in which priority is given to “the malformation components with the earliest embryonic disturbance”.15 In practice these two approaches using an “anatomical” hierarchy are broadly similar. Reports which are institution based and which cannot define the denominator population usually adopt a “physiological” hierarchy, taking as most significant that abnormality which requires the earliest intervention or which causes the most haemodynamic disturbance.4
In an effort to get round the limitations of each of these two approaches we have adopted a two dimensional classification, defining both the major structural abnormality and, where different, the abnormality precipitating clinical recognition of a cardiovascular malformation. For example, a baby with double inlet left ventricle and coarctation of the aorta would be classified by both these abnormalities, as would a baby with atrioventricular and ventriculoarterial discordance with pulmonary atresia. In both cases the former abnormality would be the more serious or fundamental malformation while the latter would precipitate presentation and diagnosis. All cases of ventricular septal defect, pulmonary stenosis or aortic stenosis were subdivided into those in which death occurred or intervention was required in infancy and those alive without intervention at 12 months. Deaths in these groups were few but in some cases the contribution of the heart defect to the death could not be ascertained retrospectively.
In order to provide larger numbers within each group for more reliable analysis of temporal variability, all cases (with the exception of patent ductus arteriosus and isolated atrial septal defect) were classified as being “complex”, “significant”, or “minor”.16 “Complex” heart disease included all cases of heterotaxy or atrial isomerism and all cases characterised by atresia or severe hypoplasia of a valve or chamber. Hearts with a common inlet valve (complete atrioventricular septal defect) or common outlet valve (truncus arteriosus) were also included in this group. Cases of “significant” cardiovascular malformation were those in which four valves and four chambers were present but where intervention was or would be required. This group, for example, includes all cases of simple transposition, tetralogy of Fallot, large ventricular septal defect, coarctation of the aorta, etc. “Minor” malformations were those where intervention would not be required. These were mainly mild or moderate aortic or pulmonary valve stenosis and smaller ventricular septal defects. Rarer malformations included in a miscellaneous group in tables 1 and 2 were also individually classified as complex, significant or minor.
Prevalence of individual cardiovascular malformations, including “anatomical” and “physiological” hierarchies
Year by year birth prevalence of individual cardiovascular malformations using and “anatomical” hierarchy
Where there were two or more malformations within the same broad diagnostic group (complex, significant or minor) the hierarchy was as shown by the order of listing in table 1. For example, a baby undergoing surgical closure of a ventricular septal defect and patent ductus was classified as having a ventricular septal defect, whereas a baby with a small ventricular septal defect requiring ligation of a ductus was classified as having a patent ductus arteriosus. As described above, isolated atrial septal defect and isolated patent ductus arteriosus were analysed separately and were not included in the complex, significant, and minor classification.
Simplified diagnostic hierarchies do not permit detailed subclassification of hearts with straddling atrioventricular valves or relative ventricular hypoplasia, features which will determine the surgical strategy and outcome but which are relatively rare. The diagnostic hierarchy does not equate with a classification into those suitable for biventricular or univentricular repair. Our anatomical classification does not include double outlet right ventricle, a diagnostic label which fails to identify an anatomically or physiologically distinct group of heart malformations.
DIAGNOSIS
Cases were included only if they were live born. In all cases the diagnosis was confirmed by echocardiography, cardiac catheterisation, surgery or necropsy. Note was made of the total infant mortality in individual diagnostic categories.
Pregnancies terminated after antenatal diagnosis of congenital heart disease were listed separately.
Any study of cardiovascular malformations in a population would ideally include all affected fetuses and would therefore include complete ascertainment of pregnancies which ended in spontaneous abortion, termination or stillbirth. There is evidence of a high prevalence of cardiovascular malformations in stillbirths but reliable ascertainment is very difficult.17 Small changes in fetal survival of some more severe malformations might be expected to have a major effect on birth prevalence.18 Spontaneous fetal losses were not included in this analysis as there is no rigorous or reliable ascertainment of cardiovascular malformations in these groups in our population.
STATISTICS
Statistical analysis was limited to analysis of trends over time in the three main diagnostic groups using a Poisson model.
Results
DENOMINATOR POPULATION
From 1985 to 1997 there were 477 960 live births, a mean of 36 766 per year. There was a 15% decline in the birth rate from 38 592 in 1991 to 32 874 in 1997, exceeding the 9% fall in births in England and Wales between 1990 and 1997.19
BIRTH PREVALENCE
In the 13 years of the study 2671 live born cases of congenital heart disease were identified, a birth prevalence of 5.6 per 1000. Table 1 shows the prevalence of individual malformations using separate “anatomical” and “physiological” hierarchies. The use of a one dimensional anatomical classification alone would lead to under ascertainment of pulmonary atresia by 28/102 (27%) cases, of interruption of the aortic arch by all 36 (100%) cases, and of coarctation of the aorta by 74/190 (39%) cases, because they would be classified only according to the most significant intracardiac malformation.
TEMPORAL VARIATION OF LIVE BORN CARDIOVASCULAR MALFORMATION
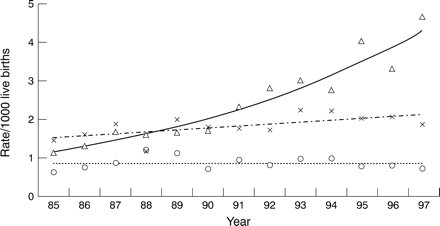
Figure 1 shows the year by year prevalence of congenital heart disease within the three main diagnostic groups. There was a highly significant trend to increase in numbers of minor malformations over time (p < 0.0001), a slight trend towards increasing numbers of significant malformations which did not reach statistical significance (p = 0.08), and no change in complex cases (p = 0.85).
Year by year prevalence at live birth of 411 complex (open circles, dotted line), 870 significant (crosses, dashed line), and 1151 minor (open triangles, solid line) cardiovascular malformations. For definitions see text.
TEMPORAL VARIATION FOR INDIVIDUAL MALFORMATIONS
Table 2 shows the year to year variability in the prevalence of individual malformations using the main “anatomical” hierarchy. Figure 2 presents year to year variability for some individual diagnoses. The greatest variation was seen in interruption of the aortic arch, double inlet ventricle, and truncus arteriosus. More variability was apparent in diagnoses with smaller numbers but there was a relatively constant birth prevalence of hypoplastic left heart despite the small numbers. Figure 2 also shows a relatively small variation in the numbers of babies with tetralogy of Fallot or transposition of the great arteries, and a wider variability in the prevalence of complete atrioventricular septal defect. There appears to be a trend towards more cases of coarctation of the aorta, despite complete ascertainment. There is a real increase in the recognition and registration of ventricular septal defects not requiring operation in infancy, but no trend in larger ventricular septal defects requiring surgery or associated with death before the age of 12 months.
Year by year prevalence at live birth of selected individual diagnoses. VSD, ventricular septal defect; CAVSD, complete atrioventricular septal defect. Data for CAVSD are divided into those with trisomy 21 (black) and without (grey).
ANTENATAL DIAGNOSIS AND TERMINATION OF PREGNANCY
One possible effect on live born prevalence of congenital heart disease is a policy of termination of pregnancy after antenatal diagnosis. Figure 3 shows the increasing number of terminations during the study period. Of 79 fetuses whose pregnancies were terminated 20 had a cardiovascular malformation associated with a chromosomal abnormality, 15 with other major malformation, and 44 had isolated heart disease. Confirmed post mortem diagnoses are shown in table3.
Year by year numbers of terminated pregnancies with congenital heart disease (CHD).
Pregnancies with congenital heart disease terminated from 1985 to 1997
Discussion
Examination of published reports on the birth prevalence of congenital cardiovascular malformations emphasises the need for a constant method in order to be able to examine apparent differences.5 ,20 There have been many reports but few meet the criteria of being population based, including only confirmed diagnoses, and limiting ascertainment to the first 12 months of life. There is also wide variation in the diagnostic hierarchies employed.4 ,14 ,15 ,21 The differences in the birth prevalence of cardiovascular malformations in different populations might be a clue to a genetic or environmental cause, but the reported variation in prevalence is almost certainly accounted for by variation in ascertainment.5 ,20 Even in large studies there are small numbers of the less common diagnoses and so no conclusions can be drawn from differences reported in the birth prevalence of rarer malformations.
This study is one of the largest to date with a denominator of nearly half a million live births. We think we have full ascertainment of “complex” and “significant” cardiovascular malformations, but there is inevitably some under ascertainment of minor malformations from the early years (1985 to 1989).
Diagnostic registers have a problem in classifying babies with multiple cardiovascular malformations. Institution based studies tend to deal with the abnormality which precipitates presentation or referral4 ,10 whereas population based studies usually rank abnormalities according to anatomical severity.14 ,15Many older studies give no indication of how multiple malformations were dealt with. Other “embryological” hierarchies have been proposed but have not gained acceptance.21 The diagnostic hierarchy presented here offers a standard and highlights the diagnoses likely to be under or over represented in studies using either anatomical or physiological hierarchies—that is, pulmonary atresia, interruption of the aortic arch, coarctation of the aorta, and total anomalous pulmonary venous connection.
This study also shows significant time related variation in the birth prevalence of cardiovascular malformations. The main reasons are better ascertainment with prospective registration, difficult retrospective ascertainment of minor malformations from the early years of the study, and increasing recognition of minor malformations using colour Doppler echocardiography, mainly of small ventricular septal defects.7 Several previous studies have documented an increase over time in the identification of more minor cardiac malformations and have attributed the change to better ascertainment.7 ,8 ,22-24 Year to year variation in the numbers of live born babies with less common malformations is quite striking (fig 2), but is almost certainly accounted for in the random variation of small numbers.
The increase in antenatal diagnosis of severe cardiovascular malformations and termination of affected pregnancies would be expected to lead to fewer cases being live born. So far it has been difficult to demonstrate this in any population based study.6 ,25 The termination of pregnancy after antenatal diagnosis of cardiovascular malformation is increasing in our catchment area, as in other regions,6 ,26 but we could show no effect of this on the prevalence at live birth of congenital heart disease. The natural history of heart disease diagnosed in utero is not clear and is obviously affected by termination of pregnancy. However, we cannot assume that those fetuses whose pregnancies were terminated would all have been live born. Fetal mortality varies considerably between diagnoses27 and there is a well recognised excess of cardiovascular malformations in stillbirths.17 ,18 In the long run the increase in antenatal diagnosis and termination of pregnancy is likely to lead to fewer live born babies with cardiovascular malformations, but so far any effect is masked by the variation in small numbers and the decline in birth rate. Pregnancies terminated will tend to be those of fetuses with the most severe, and therefore most easily recognised, malformations and with the poorest prospect of natural survival.
Within a given population there are likely to be several influences on the birth prevalence of congenital heart disease. Better ascertainment, better non-invasive diagnosis, and survival into adult life with a higher risk to offspring are likely to increase the number of diagnoses, whereas antenatal diagnosis and termination of pregnancy will reduce the prevalence at live birth of the more severe forms of heart disease. Unknown environmental influences could have either effect. The prevalence of heart defects will also obviously be influenced by the overall birth rate. When individual diagnoses are considered, year to year variations in small numbers are likely to be large making it difficult to prove that any change results from any specific intervention.
Acknowledgments
We are indebted to Professor John N S Matthews of the department of statistics, University of Newcastle for statistical analysis, to Kati Whiteoak for data collection and compilation, and to the Children's Heart Unit Fund for financial support of the paediatric cardiology database.