Article Text
Abstract
Cerebral white matter injury, characterised by loss of premyelinating oligodendrocytes (pre-OLs), is the most common form of injury to the preterm brain and is associated with a high risk of neurodevelopmental impairment. The unique cerebrovascular anatomy and physiology of the premature baby underlies the exquisite sensitivity of white matter to the abnormal milieu of preterm extrauterine life, in particular ischaemia and inflammation. These two upstream mechanisms can coexist and amplify their effects, leading to activation of two principal downstream mechanisms: excitotoxicity and free radical attack. Upstream mechanisms trigger generation of reactive oxygen and nitrogen species. The pre-OL is intrinsically vulnerable to free radical attack due to immaturity of antioxidant enzyme systems and iron accumulation. Ischaemia and inflammation trigger glutamate receptor-mediated injury leading to maturation-dependent cell death and loss of cellular processes. This review looks at recent evidence for pathogenetic mechanisms in white matter injury with emphasis on targets for prevention and treatment of injury.
Statistics from Altmetric.com
Cerebral white matter injury in the premature infant is a problem of enormous importance. For example, in the USA each year approximately 60 000 infants (1.5% of the 4 000 000 yearly live births) are born with a birth weight less than 1500 g,1 and based on MRI data at least 50% exhibit some degree of cerebral white matter injury,2 3 as defined later. This injury likely accounts for the predominance of neurological deficits observed in the approximately 90% of infants who survive. These deficits in survivors include cerebral palsy in 5–10% and importantly, cognitive/behavioural/attentional deficits in about 50%.4 5 Although other pathologies occur in premature infants—for example, severe intraventricular haemorrhage, periventricular haemorrhagic infarction, hydrocephalus, cerebellar disease—cerebral white matter injury seems to be the predominant lesion. Prevention of this injury requires insight into pathogenesis, and recent research holds promise that preventive interventions will be found.
PERIVENTRICULAR LEUKOMALACIA AND ENCEPHALOPATHY OF PREMATURITY
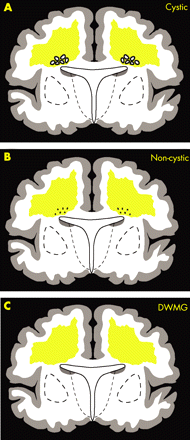
Cerebral white matter injury is the term used in this review for the full spectrum of periventricular leukomalacia (PVL). PVL has two components—that is, focal necrosis deep in the white matter with loss of all cellular elements, and a more diffuse component in central cerebral white matter with loss of pre-myelinating oligodendrocytes (pre-OLs), astrogliosis and microglial infiltration.2 PVL occurs in two overlapping forms: cystic PVL, in which the focal necroses are macroscopic and evolve to multiple cysts (fig 1A); and non-cystic PVL, in which the focal necroses are microscopic and evolve principally to glial scars (fig 1B). A third form of cerebral white matter abnormality consists of diffuse astrogliosis without focal necroses (fig 1C). That the latter is the mildest form of injury in a spectrum that includes cystic PVL as the most severe form seems likely but has not yet been established conclusively. Cystic and non-cystic PVL are most clearly related to subsequent neurological deficits. Cystic PVL is now rare; non-cystic PVL accounts for most of the cerebral white matter injury in premature infants today.2 3 6
Recently the term “encephalopathy of prematurity” has been used for the constellation of neuronal abnormalities observed in premature infants, especially those with cerebral white matter injury.7 These grey matter abnormalities, identified initially by volumetric MRI studies, include defective growth of the cerebral cortex and deep nuclear structures, in particular the thalamus and basal ganglia.6–9 A recent neuropathological study of 41 premature infants identified neuronal loss and gliosis in the thalamus in 40–55% of infants, in the basal ganglia in 30–50% and in the cerebral cortex in 10–30%.10 Importantly, the neuronal deficits were observed predominantly in brains with PVL, nearly entirely non-cystic, and were uncommon in brains with only diffuse white matter gliosis or normal white matter. The neuronal abnormalities may be particularly important in the genesis of the cognitive deficits subsequently observed in premature infants. The combination of PVL and the neuronal deficits constitutes the “encephalopathy of prematurity”.7 10 It is beyond the scope of this review to discuss the potential relationship of the white matter injury to the neuronal abnormalities—that is, whether cause–effect or association. Indeed, a fertile topic for future research is the delineation of the relationships between the involvement of the neuronal-axonal unit and the spectrum of white matter disturbance in PVL. In the remainder of this review, we focus on the pathogenesis of the white matter injury per se, with a particular focus on the pre-OL.
PATHOGENESIS OF PVL
The pathogenesis of PVL is related to a remarkable confluence of maturation-dependent factors that render the premature infant’s brain exquisitely vulnerable to the occurrence of cerebral white matter injury, as we will detail subsequently. The principal initiating pathogenetic factors in PVL appear to be cerebral ischaemia and, in a still-to-be-defined subset, maternal intrauterine (or neonatal) infection and fetal (or neonatal) systemic inflammation. These two upstream mechanisms activate two critical downstream mechanisms—that is, excitotoxicity and free radical attack by reactive oxygen and nitrogen species (ROS, RNS)—that lead to death of the vulnerable pre-OL (fig 2).
Upstream mechanisms
During peripartum development, the preterm brain is subject to intrinsic fetal and environmental factors that trigger injury to the differentiating pre-OL. The ontogenic vulnerability of the preterm brain to white matter injury together with initiating extrinsic factors are related to two broad upstream mechanisms: ischaemia and inflammation (fig 2). These mechanisms often operate simultaneously; they may act in concert to potentiate each other and are important targets in strategies to prevent or ameliorate PVL.
Ischaemia
Premature infants have a propensity for developing cerebral ischaemia, especially in white matter (fig 2). This propensity is likely because of intrinsic vascular and physiological factors, including (a) arterial border and end zones within white matter, and (b) impaired regulation of cerebral blood flow (CBF).
Vascular anatomical factors
The deep focal necrotic lesions in PVL occur in periventricular arterial end zones of long penetrating vessels derived mainly from the middle cerebral arteries. These vessels run from the pial surface and terminate in the deep periventricular white matter. The terminations of these long penetrators essentially form distal arterial fields and are most sensitive to falls in cerebral perfusion.11–13 Active development of this periventricular vasculature occurs during the last 16 weeks of human gestation.14 15 Thus, in the most immature infants, a lesser degree of ischaemia may cause focal necrotic lesions.16
The diffuse component of PVL may be similarly related to development of the more peripheral penetrating vasculature—that is, the short penetrators and the anastomoses between the long penetrators. The number of short penetrators and anastomoses between the long and short penetrators increases in the third trimester with a consequent decrease in vulnerable end zones and border zones. Recent work in fetal sheep has shown that the differential spatial and temporal distribution of pre-OLs in the developing cerebral white matter is also important in determining the regional selectivity of white matter to ischaemia.17
The functional correlate of these anatomical studies is the markedly low basal blood flow to cerebral white matter. Earlier pioneering xenon clearance studies showed low global CBF in premature infants, whereas later neonatal positron emission tomography (PET) studies showed CBF values in cerebral white matter to be startlingly low (only 25% of CBF to cortex), even in healthy preterm infants with normal neurological outcomes.18 19 The values ranged from only 1.6 ml/100 g/min to 3 ml/100 g/min, much below the threshold for viability in the adult brain (10 ml/100 g/min; normal adult CBF is 50 ml/100 g/min). This extraordinarily low CBF during early extrauterine life suggests that the survival of premature cerebral white matter depends on a marginal blood flow, and therefore may be highly vulnerable to even minor decreases in cerebral perfusion.
Impaired regulation of CBF
There is accumulating evidence that the brain of the sick preterm infant often shows impaired cerebrovascular autoregulation in response to changes in blood pressure. The cerebral circulation may become pressure-passive. The resulting inability to maintain CBF in the face of even minor falls in systemic blood pressure (as often occurs in preterm infants) might lead to ischaemia in the vulnerable arterial end and border zones described above.
Pressure-passive cerebral circulation and impaired dynamic cerebrovascular autoregulation in sick preterm infants have been shown in studies using both 133Xe clearance methods and non-invasive near-infrared spectroscopy (NIRS).20–22 In a recent series of 90 premature infants, pressure-passive periods were detected in over 95% of infants during the first 5 days of life, and the proportion of time as pressure-passive was, overall, a mean of 20%, but was in excess of 50% in some infants.23 Because only a minority of pressure-passive infants were hypotensive, it is noteworthy that under conditions of conventional neonatal intensive care the majority of infants might not be considered to be at risk for PVL.
The propensity of the preterm infant to pressure-passivity is related in part to immaturity of intrinsic vasoregulatory mechanisms, such as absence of the muscle in penetrating cerebral arterioles.24 In the stable preterm infant with intact cerebral autoregulation, clinically acceptable resting arterial pressures occur close to the lower limit of the autoregulatory curve. Thus, critical arterial pressures below which CBF begins to become pressure-passive lie dangerously close to “normal” mean arterial pressures.25 26 Clinically stable infants may thus be potentially vulnerable to impaired cerebral perfusion with small decreases in systemic blood pressure.27–29
Even when cerebrovascular autoregulation is intact, cerebral arterial end/border zones and the concentration of vulnerable pre-OLs (see later) probably ensure that premature cerebral white matter is susceptible to ischaemia in the presence of sufficient systemic hypotension or cerebral vasoconstriction. The association of PVL and severe hypocarbia or hypotension is well documented.30–34 Munro and coworkers, using NIRS, demonstrated reduced CBF in hypotensive premature babies.26 Mean arterial pressure less than 31 mmHg during the first 24 h of life in infants between 24 weeks’ and 30 weeks’ gestation was associated with impaired EEG continuity, suggesting impaired CBF during such hypotension.35 Evidence for ischaemia as a major pathogenetic mechanism emanates from a variety of animal models in which induction of decreased CBF leads to predominant white matter injury.36–38
Mechanical ventilation is often necessary in preterm infants and fluctuations in arterial carbon dioxide tension are common. Hypocarbia is a potent cerebral vasoconstrictor and an association between early hypocarbia and PVL has been described in many human studies.39–42 In a recent study of 905 infants weighing less than 1250 g, cumulative hypocarbia during the first week of life was associated with an odds ratio of 5.6 for development of PVL.30
Infection/inflammation
Infection and inflammation (including ischaemia-induced inflammation) together represent the second major upstream mechanism leading to injury or death of pre-OLs (fig 2). At the heart of this mechanism is systemic upregulation of proinflammatory cytokines and diffuse activation of microglia within immature white matter (fig 3).
Clinical studies
The fetal and neonatal human host response to injury has become a major focus of interest in understanding the pathogenesis of PVL.43 The cytokine hypothesis posits that a robust adaptive fetal systemic inflammatory response to intrauterine infection results in raised levels of blood cytokines. Although some of these cytokines are variably toxic to pre-OLs and could contribute to PVL, there is no clear correlation of neonatal plasma and cerebrospinal fluid (CSF) cytokine profiles.44 The associated microglial response in cerebral white matter with generation of free radicals may be more important (see later). It should be noted that proinflammatory cytokines are produced in response to hypoxia-ischaemia, as well as infection, further potentiating the direct effects of ischaemia.45
A number of epidemiological studies have shown an association between maternal/fetal infection and sonographically detectable PVL or cerebral palsy.43 Both outcomes are increased in the presence of infections of the decidua, placenta and amniotic fluid, fetal vasculitis, raised cytokines in neonatal blood and amniotic fluid, and intrauterine T cell activation.46 47 However, chorioamnionitis is also strongly associated with preterm delivery; controlling for gestational age frequently reduces or abolishes an association between clinical chorioamnionitis and PVL and the sequence of causation is not certain.48 In addition, most studies have shown an association between markers of infection or inflammation and the sonographically detectable cystic form of PVL, a pattern that now accounts for less than 10% of all white matter injury. Control infants with sonographically occult diffuse PVL lesions would weaken the association between infection and white matter injury. A recent prospective study of 100 preterm infants studied by MRI at term failed to find an association between moderate or severe white matter injury and chorioamnionitis.6
Box 1 Pathogenesis of periventricular leukomalacia—maturation-dependent factors in premature infants*
Upstream mechanisms
Ischaemia
Vascular anatomic factors—arterial border and end zones
Vascular physiological factors—low physiological blood flow to cerebral white matter
Pressure-passive cerebral circulation
Systemic hypotension
Hypocarbia
Infection/inflammation
Propensity for maternal intrauterine infection and fetal systemic inflammatory response
Propensity for postnatal infection
Downstream mechanisms
Free radical attack
Vulnerability of pre-OLs to free radical attack
Abundant production of both ROS and RNS in PVL (by pre-OLs, microglia, astrocytes)
Delayed development of antioxidant defences in pre-OLs
Acquisition of Fe++ by pre-OLs
Central role for microglia in free radical attack
Microglia, abundant in diffuse PVL, are potent sources of ROS/RNS
Presence of toll-like receptors on microglia; activation results in release of free radicals
Maturation-dependent concentration of microglia in normal cerebral white matter during the third trimester of human gestation
Interferon γ expression in astrocytes in diffuse PVL
Interferon γ receptor expression on pre-OLs
TNFα derived from microglia in diffuse PVL; likely interacts with interferon γ
Interferon γ toxicity greater to pre-OLs than to mature cells and potentiated by TNFα from microglia
Central role for excitotoxicity in free radical attack (see below)
Excitotoxicity
Vulnerability of pre-OLs to excitotoxicity
Exuberant expression of major glutamate transporter (source of glutamate) by pre-OLs
Exuberant expression on pre-OLs of AMPA receptors, which also are deficient in the GluR2 subunit and therefore Ca2+-permeable
Exuberant expression on pre-OLs of NMDA receptors, which also are Ca2+-permeable
Likely mechanism of excitotoxic death is generation of ROS/RNS
*Factors shown to be present in human premature brain—additional potentially relevant factors defined in experimental systems are described in the text.
The direct contribution of postnatal infection in the pathogenesis of PVL requires further study. Extremely low birthweight infants with neonatal sepsis have increased rates of cerebral palsy and PVL.49 50 A large cohort study of 6093 extremely low birthweight infants identified at least one infection during neonatal life in 65%, with rates of early onset neonatal sepsis of 15–19/1000 births.50 51 Overall, as many as 25% of all infants <1500 g will experience neonatal sepsis. Thus postnatal infection could be an important etiological factor in PVL and an important target for prevention.
Neuropathological studies
Neuropathological observations provide stronger evidence for cytokine-mediated white matter injury in the premature brain. Several cytokines (especially interferon γ and tumour necrosis factor (TNF) α) have been detected directly in human PVL lesions, expressed principally in microglia/macrophages.52 Diffuse activated microgliosis is characteristic of the diffuse PVL lesion and suggests a principal pathogenetic role for activated microglia.53
Abundant interferon γ has been detected in astrocytes in diffuse PVL, with expression correlating with the degree of evidence for oxidative attack.54 The interferon γ receptor is expressed on the surface of pre-OLs, and interferon γ is directly toxic to pre-OLs in culture, an effect potentiated by TNFα.54 55 These observations suggest an important role for both interferon γ and TNFα in the pathogenesis of PVL. Notably, Ellison and coworkers44 have demonstrated raised concentrations of TNFα in the CSF of infants with MRI-defined white matter injury.
Experimental studies
Evidence for the contribution of inflammation to preterm white matter injury emanates from studies of pregnant and neonatal animal models, involving, in particular, responses to exogenous lipopolysaccharide (LPS). These studies have identified striking upregulation of inflammatory cytokines and microglial activation.56 57 LPS activates the innate immune system through interaction with a specific toll-like receptor (TLR4) on immune cells, including microglia, and secretion of molecules directly toxic to pre-OLs (see later).58 LPS may also induce systemic hypotension, hypoglycaemia, lactic acidosis and hyperthermia, which could contribute to brain injury.36 59 60
Interaction between upstream mechanisms
Fetal and neonatal infection (and exposure to LPS or proinflammatory cytokines) can be associated with persistent systemic hypotension and impaired cerebrovascular autoregulation.61 Conversely hypoxia-ischaemia, even in the absence of infection, causes systemic and cerebral elevation of microglia-derived vasoactive cytokines such as TNFα and interleukin (IL) 1β.62 Kadhim and colleagues52 described 19 PVL cases with “asphyxia” in the background. In these cases, although cytokine immunoreactivity was consistently detected in microglia, levels were doubled in the PVL cases with systemic fetal infection compared with those without infection.
A recent study of 61 preterm infants showed increased radiological white matter injury only in infants with a history of chorioamnionitis and concurrent placental perfusion defects.63 Potentiation of infection/inflammation and ischaemia also has been shown in a number of animal studies in which pretreatment with LPS (at doses not sufficient to cause cerebral injury) caused a subthreshold hypoxic-ischemic insult to produce severe injury.64 65
Downstream mechanisms
Hypoxia-ischaemia and infection/inflammation, the two principal initiating mechanisms in pathogenesis of PVL, may act separately or in concert to activate the two principal downstream mechanisms, excitotoxicity and free radical attack by ROS/RNS (fig 2).66 Excitotoxicity likely leads to pre-OL injury by promoting Ca2+ influx and, as a result, generation of ROS/RNS. However, it remains unproved whether excitotoxicity occurs entirely via generation of free radicals. Moreover, because free radicals are generated by mechanisms other than excitotoxicity—for example, microglial activation, we will consider excitotoxicity and free radical attack separately. The critical role of a series of maturation-dependent factors that underlie the particular vulnerability of cerebral white matter of the premature infant to injury will be a recurring theme (box 1).
Vulnerability to free radical attack
Evidence for free radical attack in PVL
The most compelling direct evidence that free radical attack by both ROS and RNS is involved in the injury to pre-OLs in PVL comes from the study of the human lesion.53 67 In our study of 17 cases, in which immunocytochemical markers for oxidative (hydroxynonenal) and nitrative (nitrotyrosine) attack were used, abundant staining was documented in both pre-OLs and reactive astrocytes in the diffuse lesion.53 The free radical attack seemed to lead to death of pre-OLs but not of the reactive astrocytes. This key discovery of oxidative and nitrative attack in PVL is consistent with experimental data indicative of66 68:
oxidative and nitrative cellular injury with both hypoxic-ischemic and inflammatory insults to brain, the two likely key upstream mechanisms in PVL;
an exquisite vulnerability of pre-OLs to ROS/RNS (fig 2).
Studies of CSF in living premature infants also support toxicity of ROS in PVL. Thus, in a longitudinal study of premature infants, CSF levels of oxidative products (detected as protein carbonyls), measured earlier in the neonatal period, were sharply raised in those with MRI evidence for PVL at term, compared with CSF levels in premature infants without later MRI evidence for PVL.69
Mechanisms for vulnerability of pre-OLs to ROS attack
The mechanisms underlying the maturation-dependent vulnerability of pre-OLs to ROS attack have been addressed in both human brain and experimental studies. The findings in human brain indicate a delay in development of enzymes at the superoxide dismutase (SOD) step, both MnSOD and Cu/ZnSOD, and catalase (box 1).70 In addition, the possibility that hydrogen peroxide accumulates, and in the presence of iron is converted to the hydroxyl radical by the Fenton reaction is suggested by the observations of the early appearance of iron in developing human white matter71 72 and the acquisition of iron by developing oligodendrocytes for differentiation.73 Supportive of a relationship between iron and PVL is the demonstration in premature infants that for many weeks after intraventricular haemorrhage, a disorder that sharply increases the risk of PVL,74–76 CSF levels of non-protein-bound iron are markedly increased.77 Taken together, the findings indicate a maturation-dependent window of vulnerability to oxidative attack during oligodendroglial development related principally to delayed development of antioxidant enzymes and acquisition of iron for differentiation.
Mechanisms for vulnerability of pre-OLs to RNS attack
The mechanisms underlying the maturation-dependent vulnerability of pre-OLs to RNS attack have been addressed, especially in studies of cultured cells.78 79 Relevance of these mechanisms to human PVL is provided by our preliminary data which show a significant increase in the number of inducible nitric oxide synthase (iNOS)-positive glia in the diffuse component of PVL, especially in the reactive astrocytes in the diffuse lesion.55 The data suggest that a key source of nitric oxide in the human lesion, potentially leading ultimately to a major portion of the nitrative stress identified previously in diffuse PVL,53 is the reactive astrocyte. However, it is likely that the superoxide anion necessary for combination with nitric oxide to form the injurious RNS, peroxynitrite, is derived from both the abundant activated microglia in diffuse PVL and the pre-OL itself. 78–80 As noted earlier there is a relative deficiency of both SODs in pre-OLs in cerebral white matter of the human premature infant.
Central role for microglia in ROS/RNS attack
Microglia may play a central role in the generation of ROS/RNS species involved in PVL, and this role may be greatest under conditions of the combination of ischaemia and infection/inflammation (fig 3). As discussed earlier, a potentiating interaction between systemic infection/inflammation and hypoxia-ischaemia in the genesis of cerebral white matter injury is likely.64 81–83 Microglia may have a pivotal role in this interaction, and the deleterious effect of activation of microglia in this context likely involves the generation of ROS/RNS. Recent study of human PVL has shown a marked microgliosis in the diffuse component of PVL.53 The discovery of toll-like receptors on brain microglia and their involvement in activation of microglia and production of diffusible RNS toxic to pre-OLs suggest that innate immunity can be involved in the pathogenetic cascade.64 66 81 Taken together the findings suggest that the two potent activators of microglia, hypoxia-ischaemia and infection/inflammation, converge on the microglial cell to provoke a deleterious series of effects leading to secretion of toxic products, especially ROS/RNS, and pre-OL death.
Cytokines released by activated microglia may also have a major role in the pre-OL toxicity, at least in part via RNS (fig 3). Thus, in one series (n = 19) TNFα was shown to be abundant in PVL lesions, all of which were associated with “asphyxia”, and levels were still greater when evidence for fetal inflammation or neonatal infection was also present.52 TNFα leads to pre-OL toxicity in part by potentiating the toxicity of interferon γ, and the toxicity of both of these cytokines is maturation-dependent—that is, greater to pre-OLs than to mature oligodendrocytes.84–87 The major sources of interferon γ may be astrocytes, which contain the cytokine in abundance in diffuse PVL, and the major target of its toxicity could be the pre-OL, which expresses the interferon γ receptor.54 Induction of iNOS and thereby RNS appears to be the principal mode of cell death induced by interferon γ.88
The particular involvement of microglia in the pathogenesis of cerebral white matter disease in the premature infant may relate also to a recently discovered maturation-dependent feature. Thus, it is noteworthy that microglial cells can be identified in normal human brain very early in development, become abundant in forebrain from 16 to 22 weeks of gestation and notably are concentrated in cerebral white matter, with a deep to superficial gradient.89–92 Relatively few microglia are found in cerebral cortex at this time. In a recent longitudinal study of human postmortem brain, density of microglia in white matter reached a peak during the period of greatest vulnerability to PVL (third trimester of gestation) and declined markedly in white matter after 37 weeks of gestation.92 This observation suggests that a wave of perhaps migrating microglia is present in cerebral white matter at the optimum time for activation by hypoxia-ischaemia or infection or both. Thus, although more data on these developmental features are needed, a maturation-dependent population of cells—that is, microglia—may be concentrated in cerebral white matter at the right time and in the right place to contribute to white matter injury when activated.
Excitotoxicity
Vulnerability of pre-OLs to excitotoxicity
An intrinsic maturation-dependent vulnerability of pre-OLs to excitotoxicity is suggested by recent experimental studies and now by related observations of developing human brain (see later).66 Glutamate is capable of inducing maturation-dependent death of pre-OLs by non-receptor and receptor-mediated mechanisms (fig 2). The non-receptor-mediated mechanism involves glutamate competition for the cystine transporter and promotion of cystine efflux under conditions of high extracellular levels of glutamate.93–95 The result is depletion of intracellular glutathione (which requires cysteine for biosynthesis) and cell death by oxidative stress. However, the substantial levels (millimolar) of glutamate required for this effect suggest that this mechanism may not operate in vivo under most pathological conditions. By contrast the receptor-mediated mechanism, which requires micromolar levels of glutamate, is more likely to occur in vivo, as shown directly in animal models by us and others (see later).
Sources of glutamate
The principal sources of elevated extracellular glutamate in cerebral white matter with hypoxia-ischaemia are glutamate transporters.96 97 Failure of glutamate uptake and actual reversal of transport occurs in the setting of energy failure because of the failure of the Na+/K+ ion pump. These transporters are high-affinity, sodium-dependent systems. That this transport disturbance may occur in the human infant is suggested by the recent discovery of a maturation-dependent overexpression in cerebral white matter of the principal glutamate transporter during the peak period of PVL.98 The principal locus for this transporter is the pre-OL.98 Axons may be a second major source of glutamate under conditions of hypoxia-ischaemia.99 In addition, with inflammation activated microglia also release glutamate via reversal of a Na+-dependent transporter, operation of the cystine-glutamate antiporter and vesicular release (fig 3).97 Additional links of altered glutamate homeostasis to inflammation include the potent inhibition of glutamate transport in multiple cell types by TNFα and IL1β.100 101
Elevations of glutamate in vivo
Elevations of extracellular glutamate have been documented in cerebral white matter in a sheep hypoxia-ischaemia model of PVL.102 The extent of the increase in glutamate correlated directly with the ultimate extent of the white matter injury. The major increase in glutamate occurred over the hours after the insult, and this delayed increase is characteristic of models in which an effect on glutamate transport is responsible.
Receptor-mediated excitotoxicity
Receptor-mediated glutamate toxicity is the principal mode of excitotoxicity and only in the past decade has it become clear that pre-OLs contain glutamate receptors which, when excessively activated, lead to cell injury. The most widely studied glutamate receptor in pre-OLs, the AMPA/kainate (AMPA/KA)-type, is concentrated in cell somata and leads to cell death when excessively activated (see later). The more recently discovered type, the NMDA receptor, is concentrated in oligodendroglial processes and leads to loss of cell processes when excessively activated. We discuss both these receptors next.
Oligodendrocytes express AMPA/KA-type glutamate receptors, the activation of which results in cell death.96 103–106 Our study of excitotoxicity in the major phases of the oligodendroglial lineage in culture showed that the toxicity is maturation-dependent and that both functional activity and subunit expression of AMPA/KA receptors are upregulated in pre-OLs rather than in mature oligodendrocytes.104 107 Relevance of these findings to hypoxia-ischaemia was suggested by the demonstration in culture by others and by us that receptor-mediated excitotoxicity is the principal mechanism for pre-OL death with oxygen-glucose deprivation (OGD), an in vitro model of ischaemia.108–111 Relevance to hypoxia-ischaemia in vivo was shown after the development of a rodent model of hypoxia-ischaemia-induced PVL (P-7 rat subjected to unilateral carotid ligation and hypoxaemia).37 Importantly, this white matter injury could be prevented by systemic administration, beginning immediately post-insult, of NBQX, an AMPA/KA antagonist.37 NBQX may not be clinically safe, and in subsequent work topiramate, a clinically safe anticonvulsant drug with AMPA blocking properties, was shown to also have a similar protective effect.112 Additional support for a relationship between hypoxia-ischaemia, excitotoxicity and PVL comes from the observation that AMPA receptors are overexpressed (relative to the mature brain) in white matter of developing rats during the peak period of vulnerability of this species (ie P7) for selective, hypoxic-ischemic white matter injury (see earlier).113
The mechanism of the receptor-mediated toxicity appears to involve Ca2+ influx.103 107–110 113–115 The basis for the Ca2+ influx relates to the expression in developing versus mature oligodendrocytes of AMPA receptors which lack the GluR2 subunit, the subunit that renders the receptor Ca2+ impermeable.103 108 112 Relevance to PVL is suggested by the observation that in human brain, not only are AMPA receptors also overexpressed in cerebral white matter in pre-OLs during the peak period of vulnerability to PVL, but these receptors also are relatively deficient in the GluR2 subunit and thereby Ca2+-permeable. 112 116
A major advance in the understanding of oligodendroglial excitotoxicity was the recent discovery of NMDA receptors on processes of oligodendrocytes, from the developing to the mature, myelin-producing stages.106 117–120 When activated by ischaemic conditions or exposure to agonists, loss of processes occurs. Moreover, NMDA receptors are permeable to calcium, and it is likely that downstream mechanisms related to Ca2+ influx—that is, generation of ROS/RNS, account for the deleterious effects. Because axons can release glutamate the findings suggest that the presence of NMDA receptors on oligodendrocytes provide a physiological mechanism of axonal-oligodendroglial signalling important for myelination. However, with excessive glutamate, as occurs with ischaemia, this normal mechanism becomes pathological. Importantly, the data suggest an additional site for protection of pre-OLs from ischaemic injury. Indeed, in preliminary data our group has shown a potent protective effective of memantine, a specific NMDA antagonist, in a neonatal rat model of hypoxic-ischemic white matter injury.121 Moreover, preliminary studies of human brain show, analogous to the findings with AMPA receptors, a marked expression of NMDA receptors in pre-OLs in human cerebral white matter during the peak period of vulnerability to PVL (Talos et al., 2007, manuscript in preparation). The findings of NMDA receptors on pre-OLs may help explain the white matter injury produced in developing mice and rabbits by intracerebral injection of ibotenic acid and NMDA, both agonists of the NMDA receptor.122–125 However, because NMDA receptors are also present on microglia and astrocytes, secondary effects involving pre-OLs could account for some of the white matter injury in these models. At any rate, the findings of an overexpression of both Ca2+-permeable AMPA receptors and NMDA receptors on pre-OLs suggest a particular maturation-dependent vulnerability of these cells to excitotoxicity (box 1).
Relation of excitotoxicity to ROS/RNS toxicity
Box 2 Selected potential preventive interventions for periventricular leukomalacia*
Upstream mechanisms
Ischaemia
Detection of pressure-passive cerebral circulation
Avoidance of hypotension
Avoidance of hypocarbia
Infection/inflammation
Antenatal corticosteroids
Downstream mechanisms
Free radical attack
Prevention of free radical generation
Oxygenase inhibitors (indometacin, 12-lipo-oxygenase inhibitors)
Inhibitors of nitric oxide synthase
Vitamin K
Antimicroglial agents—minocycline
Replenish antioxidant defences
Antioxidant enzyme mimetics
Scavenge free radicals
Vitamin E
Idebenone, N-acetylcysteine, other drugs
Excitotoxicity
Block AMPA receptors
Topiramate
Block NMDA receptors
Memantine
Anti-apoptotic agents
Oestradiol
Insulin-like growth factor-1
Other agents – multiple downstream effects
Melatonin
Erythropoietin
Caffeine
*A partial list of potential preventive interventions.
A direct relation of the two downstream mechanisms leading to death of the pre-OL—that is, excitotoxicity and ROS/RNS toxicity (fig 2)—is suggested by the recent demonstrations that AMPA/KA receptor toxicity to oligodendroglial precursors is accompanied by generation of ROS and RNS.110 115 126 The occurrence of nitrotyrosine immunoreactivity in the pre-OLs suggested that under these conditions peroxynitrite is involved. This deadly compound is probably formed from nitric oxide, produced by Ca2+-activated inducible NOS, and superoxide anion, produced by one or more of several Ca2+-inducible enzymes resulting in ROS formation.126 Notably the oxidative stress associated with AMPA receptor activation in oligodendrocytes is greater than that associated with such activation in neurons.127 Importantly, use of a superoxide dismutase/catalase mimetic, Euk, protected pre-OLs from OGD-induced excitotoxicity.110 This small non-peptidyl molecule can penetrate the blood–brain barrier and has neuroprotective properties in vivo.
CONCLUSIONS
Data indicate how multiple maturation-dependent characteristics of pre-OLs interact in an amplifying manner to produce a highly vulnerable cell, with the upstream mechanisms, hypoxia-ischaemia and inflammation, converging on two interacting downstream mechanisms, excitotoxicity and free radical attack (fig 2). Interruption of these mechanisms has been shown in experimental models to lead to prevention or amelioration of white matter injury. A selected summary of the most promising interventions is provided in box 2. Limitations of space preclude a detailed discussion and listing of primary references, available elsewhere.66 Nevertheless, it is now clear that the extraordinary insights into pathogenesis of cerebral white matter injury in recent years has provided considerable hope that prevention of this common and serious form of brain injury of the premature infant may soon be possible.
REFERENCES
Footnotes
Funding: The personal research described in this review was supported by grant P01 NS 38475 (JJV).
Competing interests: None.
Linked Articles
- Fantoms