Article Text
Abstract
Context In spontaneously breathing preterm infants with respiratory distress syndrome (RDS) receiving nasal continuous positive airway pressure, a method of less invasive surfactant administration (LISA) using a thin catheter has been described as an alternative to endotracheal intubation for surfactant delivery to reduce lung injury.
Objective A systematic review of randomised controlled trials (RCTs) comparing LISA with the standard method of surfactant delivery for clinical outcomes.
Methods Medline, CENTRAL and Embase databases were searched (until 29 October 2015). Additional citations were identified from trial registries, conference proceedings and the bibliographies of selected articles. The included studies were RCTs enrolling preterm infants with RDS and compared LISA technique with intubation for surfactant delivery for any of the prespecified clinical outcomes.
Results Six RCTs were identified, enrolling a total of 895 infants. The use of LISA technique reduced the composite outcome of death or bronchopulmonary dysplasia (BPD) at 36 weeks (risk ratio (RR)=0.75 (95% CI 0.59 to 0.94), p=0.01), BPD36 among survivors (RR=0.72 (0.53 to 0.97), p=0.03), need for mechanical ventilation within 72 hours of birth (RR=0.71 (0.53 to 0.96), p=0.02) or need for mechanical ventilation anytime during the neonatal intensive care unit stay (RR=0.66 (0.47 to 0.93), p=0.02). There were no differences noted for the outcome of death and other neonatal morbidities. Procedure failure rate on the first attempt and the need for additional doses of surfactant were not different between the intervention groups.
Conclusions LISA technique for surfactant delivery results in a lesser need for mechanical ventilation in infants with RDS, reduction in the composite outcome of death or BPD at 36 weeks, and BPD36 among survivors.
- less invasive surfactant administration
- systematic review
- InSurE
- respiratory distress syndrome
- meta-analysis
Statistics from Altmetric.com
- less invasive surfactant administration
- systematic review
- InSurE
- respiratory distress syndrome
- meta-analysis
What is already known on the topic?
Pulmonary surfactants reduce the risk of death and bronchopulmonary dysplasia (BPD) in preterm infants with respiratory distress syndrome.
Lungs of preterm infants are susceptible to injury from mechanical ventilation.
In preterm infants stabilized on nasal CPAP, less invasive surfactant administration techniques that avoid mechanical ventilation may further reduce the risk of death or BPD.
What this study adds?
Less invasive surfactant administration reduces the composite outcome of death or bronchopulmonary dysplasia, need for mechanical ventilation, and BPD36 among survivors.
Background
Respiratory distress syndrome (RDS) is a common neonatal condition in premature infants. Its treatment often requires the use of surfactants, which have been shown to reduce the risk of death and bronchopulmonary dysplasia (BPD) in this population.1 ,2 The most common technique for surfactant delivery currently involves endotracheal intubation and short-duration mechanical ventilation. However, the lungs of premature infants are particularly susceptible to ventilator-induced lung injury.3–7 The use of non-invasive ventilation with nasal continuous positive airway pressure (CPAP) has been shown to cause less alveolar injury compared with mechanical ventilation via endotracheal tube.8–10
Currently, the preferred strategy for management of RDS is nasal CPAP at onset with selective use of surfactant for those infants with increasing oxygen requirements.11–14 Infants meeting the criteria for surfactant use are intubated and briefly ventilated for surfactant delivery by a protocol often referred to as InSurE (Intubation, Surfactant administration and Extubation).15–18
To prevent intubation for surfactant delivery in preterm infants with RDS, less invasive surfactant administration (LISA) techniques have been described.19–22 Of these techniques, the use of a thin catheter for intratracheal surfactant delivery in spontaneously breathing preterm infants on nasal CPAP is the most studied,23 with proposed benefits in terms of better survival and decreased need for mechanical ventilation.24
Our objective was to conduct a systematic review of randomised controlled trials (RCTs) comparing LISA technique using a thin catheter with endotracheal intubation for surfactant delivery for pertinent neonatal outcomes.
Methods
Search strategy
The research librarian in collaboration with the research team conducted structured searches on subject headings and keywords for concepts related to surfactant application in preterm infants (see online supplementary appendix 1). The initial search was conducted in the first week of September 2014 employing the following electronic databases: Ovid Medline (1946 to date), Cochrane Central Register of Controlled Trials (CENTRAL) via Cochrane Library (from inception to date) and Ovid Embase (1980 to date). The search was updated on 29 October 2015. Additional citations were identified from trial registries (clinicaltrials.gov, http://www.who.int/ictrp/en/), conference proceedings (2011–2015 abstracts of annual meetings of Pediatric Academic Societies and European Society for Pediatric Research) and the bibliographies of selected articles.
supplementary appendix 1
Study selection and data extraction
Two reviewers independently searched for eligible studies and the discrepancies were resolved through discussion with a third reviewer. Studies were included if they met the following criteria: randomised sequence generation, compared LISA technique with the standard technique of surfactant delivery involving endotracheal intubation and measured one or more of the following outcomes: death and/or BPD at 36-week gestation, need for mechanical ventilation or any other neonatal morbidities. Data were extracted by one reviewer using a standardised form and checked independently for accuracy by two other reviewers. Primary authors of the included studies were contacted for clarifications and additional information, if needed. Our primary outcome was a composite of death prior to 36-week gestation or BPD36.
Assessment of bias
We addressed methodological quality as per the Cochrane risk of bias tool,25 which includes items for adequacy of random sequence generation, allocation concealment, blinding of the caregivers and the assessors, incomplete outcome data reporting or selective reporting. Discrepancies were resolved through discussion and consensus among the review team.
Data analysis
Studies were pooled using random effects model.26 Risk ratio (RR) with its 95% CI was chosen as the principal summary measure for assessing treatment effect. Data were analysed using RevMan V.5.3. An a priori sensitivity analysis was planned for studies that explicitly stated InSurE technique as the method of surfactant delivery for control group. The study is reported according to the PRISMA guidelines (http://www.prisma-statement.org) (see online supplementary appendix 2). I2 statistic was calculated for each analysis to quantify heterogeneity across studies. If substantial (I2>50%) heterogeneity was detected, the potential causes for its existence were explored and further sensitivity analyses were undertaken.
supplementary appendix 2
Results
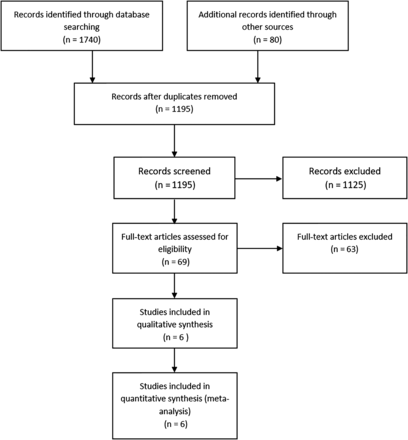
Figure 1 shows the flow of the studies through the selection process. We identified six RCTs, enrolling a total of 895 preterm infants.27–32 Brief descriptions and main characteristics of the included trials are presented in table 1. Curosurf was the predominant surfactant used in the included studies. The use of antenatal steroids was similar in the LISA and the control groups for each study. The data on caffeine (or other methylxanthines) use were available from five trials. Three trials prescribed these agents to nearly all participants,27 ,28 ,32 and the other two trials restricted the use of methylxanthines according to gestation (all participants <32 weeks’ gestational age)29 or birth weight (all participants <1250 g).31 The starting time for these agents was not provided for comparison, although Kribs et al32 stated that the LISA group participants received caffeine earlier than controls who received caffeine at extubation. The use of postnatal steroids was reported in only two trials27 ,32 and was comparable in the intervention groups.
Baseline characteristics of the included studies
A PRISMA flow chart for the selection of eligible studies. BLES, bovine lipid extract surfactant; LMA, laryngeal mask airway; nCPAP: nasal CPAP; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
In all studies, the enrolled subjects were initiated on nasal CPAP (at 4–8 cm of H2O pressure) for management of RDS prior to randomisation. The criteria for providing surfactant were comparable between the intervention groups (ie, similar threshold of fractional inspired oxygen (Fio2) and/or severity of respiratory distress in both groups) in all but one trial.27 Göpel et al27 used Fio2 of >30% as a criterion for surfactant delivery in the LISA group, whereas in the control group the Fio2 threshold for surfactant use was allowed as per site-specific guidelines. However, this did not lead to a significant difference between the two groups in terms of the Fio2 requirements at the time of surfactant delivery (median (IQR) Fio2: 40% (35%–55%) in the LISA group vs 45% (40%–60%) in the control group), as well as the proportion of subjects requiring surfactant (74% vs 65%, p=0.19).
Risk of bias assessment
Table 2 shows the risk of bias assessment of the included studies. Three studies28 ,29 ,31 did not adequately describe the method of randomisation and two studies29 ,30 did not describe the method of allocation concealment. As expected, no blinding of interventions in any of the studies was attempted (technically difficult to perform given the type of interventions). This could have resulted in bias for some important outcomes such as ‘need for mechanical intubation’. However, all studies satisfactorily described their objective criteria for rescue intubation to minimise this bias. Göpel et al27 used somewhat different criteria for the surfactant delivery in the two intervention groups (as explained above).
Risk of bias assessments
Outcomes
Death or BPD at 36 weeks
All studies provided data for our primary outcome. A meta-analysis showed that the LISA technique resulted in a significant reduction in the composite outcome of death or BPD at 36 weeks (RR=0.75 (95% CI 0.59 to 0.94), p=0.01) (figure 2). A sensitivity analysis restricted to studies clearly stating the use of the InSurE protocol for control groups showed similar results (four studies,28–31 RR=0.66 (95% CI 0.46 to 0.94), p=0.02). An analysis restricted to studies with the lowest risk for bias revealed similar results (two studies,28 ,32 RR=0.75 (95% CI 0.57 to 1.00), p=0.05). There was no significant heterogeneity noted in any of these analyses.
Composite outcome of death or bronchopulmonary dysplasia at 36 weeks. LISA, less invasive surfactant administration.
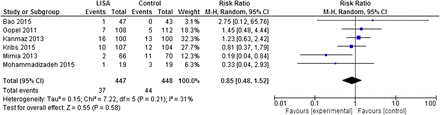
Death
The meta-analysis showed no difference for the outcome of death (RR=0.85 (95% CI 0.48 to 1.52), p=0.58) (figure 3). The sensitivity analysis restricted to trials28–31 explicitly stating the use of InSurE protocol in control arms revealed similar results.
A meta-analysis of the outcome of death in study participants. LISA, less invasive surfactant administration.
BPD36 among survivors
The meta-analysis showed that the LISA technique resulted in a significant reduction in this outcome (RR=0.72 (95% CI 0.53 to 0.97), p=0.03) (figure 4). The sensitivity analysis restricted to trials stating the use of InSurE protocol in the control arms28–31 revealed similar point estimate for treatment effect (RR=0.70 (95% CI 0.42 to 1.16), p=0.16). The analysis restricted to studies with the lowest risk for bias revealed similar results (two studies,28 ,32 RR=0.70 (95% CI 0.48 to 1.03), p=0.07).
Bronchopulmonary dysplasia at 36 weeks among survivors. LISA, less invasive surfactant administration.
Mechanical ventilation
Four studies28–31 presented data for the outcome of need for mechanical ventilation within 72 hours of birth, and three studies27 ,28 ,32 for the outcome of need for mechanical ventilation anytime during hospitalisation. The meta-analysis showed that the LISA group infants had a lesser need for mechanical ventilation within 72 hours (RR=0.71 (95% CI 0.53 to 0.96), p=0.02) (figure 5), and for mechanical ventilation anytime during the neonatal intensive care unit (NICU) stay (RR=0.66 (95% CI 0.47 to 0.93), p=0.02) (figure 6). The latter analysis showed significant heterogeneity (I2=85%) resulting from more favourable results seen in the trial by Göpel et al27 compared with those seen in the other trials. When data from this study were excluded the heterogeneity resolved (I2=0%), and the pooled estimate remained significant (RR=0.76 (95% CI 0.65 to 0.85), p<0.001). The beneficial effect with LISA remained when analyses were restricted to studies28 ,32 with the lowest risk for bias.
Need for mechanical ventilation within 72 hours from birth. LISA, less invasive surfactant administration.
Need for mechanical ventilation anytime during the neonatal intensive care unit stay. LISA, less invasive surfactant administration.
The included studies also presented data for the duration of mechanical ventilation in the intervention groups (table 3). However, those data were in variable units, preventing pooling of results for a meta-analysis.
Duration of mechanical ventilation in the intervention groups for the included studies
Other neonatal morbidities
The meta-analysis showed lesser pneumothoraces in the LISA group infants (five studies,27–30 ,32 RR=0.61 (95% CI 0.37 to 1.02), p=0.06, I2=0%). Similarly, a non-significant reduction in the incidence of pulmonary haemorrhage was noted with LISA (three studies,27 ,28 ,32 RR=0.63 (95% CI 0.29 to 1.37), p=0.24). No significant differences were noted in outcomes of other neonatal morbidities, that is, patent ductus arteriosus requiring medical or surgical treatment, necrotising enterocolitis ≥stage 2, retinopathy of prematurity >stage 2, intraventricular haemorrhage >stage 2 and periventricular leukomalacia (table 4).
Results of all outcomes studied
Procedure-related outcomes
There was a greater incidence of surfactant reflux noted with the LISA technique (three studies,28–30 RR=2.52 (95% CI 1.47 to 4.31), p<0.001). However, this did not affect the number of subjects needing more than one dose of surfactant in the intervention groups (four studies,28–31 RR=1.07 (95% CI 0.80 to 1.44), p=0.63). The outcome of failure of procedure on the first attempt was not different between the groups (four studies,28 ,30–32 RR=0.97 (95% CI 0.58 to 1.63), p=0.91).
Two trials31 ,32 reported increased incidence of bradycardia and/or desaturations during the LISA procedure, while Bao et al30 reported a lesser incidence of these events in the LISA group. Kanmaz et al28 reported no such differences between the study groups.
Discussion
The results of this systematic review of available RCTs show that in non-invasively ventilated preterm infants, the use of the LISA technique compared with that of endotracheal intubation for surfactant delivery was beneficial in terms of reduction in the composite outcome of death or BPD at 36 weeks, BPD36 among survivors and the need for mechanical ventilation. There was also a trend towards lower pneumothorax rates with LISA. These findings were robust to the sensitivity analyses performed. There were no differences noted in the outcome of death or in the rates of other neonatal morbidities. We included the study by Göpel et al27 where not all infants enrolled in the trial received surfactant (only those who met the criteria received surfactant), as the proportion of infants who received surfactant and the Fio2 requirement at the time of surfactant delivery were not statistically different between the two study arms. In the sensitivity analysis, the exclusion of data from this trial did not affect the main findings of this review.
We restricted our analysis to the LISA technique that involved surfactant delivery using a thin catheter. Other methods of surfactant delivery without intubation such as administration by nebulisation or with the use of LMA have been described in literature.21 ,33–35 We did not evaluate those techniques as the literature on them is currently limited.
The pulmonary benefits seen with the LISA technique may be related to many factors. First, a few large mechanical breaths, which are often required with the current method of surfactant delivery following intubation, could cause lung injury in the early newborn period as described in animal studies.5 Second, the LISA technique allows for continuation of uninterrupted nasal CPAP support during the entire process of providing surfactant, preventing lung injury that could result from the temporary loss of functional lung capacity and atelectasis during the process of intubation.36 Lastly, the LISA technique largely depends on the spontaneous breathing effort of the newborns to distribute surfactant in the lungs, compared with the repeated positive pressure inflations with the InSurE technique, resulting in more rapid and complete tissue incorporation of the surfactant in the neonatal lung.37 ,38
The results of this systematic review suggest that the LISA technique should be the preferred method for delivering surfactant to spontaneously breathing preterm infants in the neonatal units. The findings of our systematic review differ from those of a meta-narrative review23 published previously, which concluded no significant difference in the outcome of BPD with the LISA technique. However, many new RCTs30–32 published since then were included in our meta-analyses, allowing us to provide more precise estimates for the neonatal outcomes studied. A recently published large cohort study, enrolling over 2200 preterm infants,39 confirms the findings of our systematic review.
The need for premedication with the LISA procedure was inconsistently reported in the included trials. It is difficult to comment from the available data whether the use of premedication would help reduce the incidence of bradycardia and/or desaturation episodes during the LISA procedure, as noted in some studies.31 ,32 A recent cohort control study showed better comfort scores with the use of sedation but a greater risk of intubation during or within 24 hours after the procedure.40 Future studies could determine the benefits and the risks associated with the use of sedation during the LISA procedure.
The widespread implementation of LISA technique in the neonatal units would require formal training of healthcare personnel for this procedure. Several variations of the LISA technique have been described, such as the use of feeding tubes or semirigid vascular catheters, with or without the aid of Magill forceps. Further research would be needed to identify the most suitable technique for wider adoption.
Limitations
Our systematic review has a few limitations. None of the included trials provided data regarding long-term neurodevelopmental outcomes. However, a recent cohort study using historical controls showed no difference in long-term outcomes at school age with LISA.41 Two large multi-centre studies27 ,32 did not clearly mandate the use of the InSurE protocol in control groups. However, study investigators for both of these trials suggested extubation as soon as possible to the participating sites as expected with the InSurE protocol. The sensitivity analysis excluding data from those trials did not change the results of our primary outcome.
The included studies did not systematically report procedure-related side effects. However, the studies that presented these data did not reveal a greater failure rate with the LISA procedure on first attempt28 ,30–32 or the need for additional doses of surfactant.28–31
The majority of subjects enrolled in the included studies received a porcine surfactant (Curosurf). It is possible that the procedure-related side effects such as surfactant reflux, bradycardia and/or desaturation events may occur more frequently with bovine surfactants (BLES and Survanta) where the volume required is usually greater. A few ongoing RCTs (ECALMIST NCT01848262, MISTCPAP NCT01723683 and MIsurf NCT01615016) using bovine surfactants may answer this question with greater certainty in the coming years.
Conclusions
In spontaneously breathing preterm infants with RDS stabilised on nasal CPAP, the LISA technique for surfactant delivery resulted in less need for mechanical ventilation during the NICU stay and a reduction in the composite outcome of death or BPD at 36 weeks and BPD36 among survivors. There were no clinically significant side effects noted. For a widespread implementation of the LISA technique, training of healthcare personnel, standardisation of the procedure including the role of premedication and more experience with extremely preterm infants are recommended.
References
Footnotes
Contributors JCA-A contributed to all stages of the review, wrote the first draft of the manuscript and approved the final manuscript as submitted. MP contributed to the planning of the study, literature search, data extraction and risk of bias assessments, and approved the final manuscript as submitted. RMF contributed to the planning of the study and literature search, and approved the final manuscript as submitted. MK contributed to all stages of the review, reviewed all drafts of the manuscript and approved the final manuscript as submitted.
Competing interests None declared.
Linked Articles
- Fantoms