Article Text
Abstract
Objective To evaluate the effect of implementing automated oxygen control as routine care in maintaining oxygen saturation (SpO2) within target range in preterm infants.
Methods Infants <30 weeks gestation in Leiden University Medical Centre before and after the implementation of automated oxygen control were compared. The percentage of time spent with SpO2 within and outside the target range (90–95%) was calculated. SpO2 values were collected every minute and included for analysis when infants received extra oxygen.
Results In a period of 9 months, 42 preterm infants (21 manual, 21 automated) were studied. In the automated period, the median (IQR) time spent with SpO2 within target range increased (manual vs automated: 48.4 (41.5–56.4)% vs 61.9 (48.5–72.3)%; p<0.01) and time SpO2 >95% decreased (41.9 (30.6–49.4)% vs 19.3 (11.5–24.5)%; p<0.001). The time SpO2<90% increased (8.6 (7.2–11.7)% vs 15.1 (14.0–21.1)%; p<0.0001), while SpO2<80% was similar (1.1 (0.4–1.7)% vs 0.9 (0.5–2.1)%; ns).
Conclusions During oxygen therapy, preterm infants spent more time within the SpO2 target range after implementation of automated oxygen control, with a significant reduction in hyperoxaemia, but not hypoxaemia.
- Oxygen
- Hypoxaemia
- Hyperoxaemia
- Preterm infant
Statistics from Altmetric.com
What is already known on this topic?
The frequency and duration of hypoxaemia and hyperoxaemia in preterm infants influence survival and long-term outcome.
Titrating oxygen manually to maintain oxygen saturation within a narrow target range can be challenging.
Randomised trials have shown that automated oxygen control is effective, but this has only been measured for short periods.
What this study adds?
After implementation of automated oxygen control for daily care, preterm infants spent more time with their oxygen saturation within the target range.
After implementation, hyperoxaemia significantly decreased during oxygen therapy, but there was no effect on hypoxaemia.
Introduction
To prevent hypoxaemia and hyperoxaemia in preterm infants, nurses manually titrate the fraction of inspired oxygen (FiO2) in order to maintain pulse oximeter saturation (SpO2) within a set target range. Studies have shown that compliance with SpO2 targets is low and there is a tendency for nurses to accept higher SpO2.1–5 Manual titration of oxygen is challenging, especially during hypoxaemic and bradycardic events related to apnoea of prematurity.6 ,7 We recently demonstrated that manual titration of oxygen therapy in preterm infants during these hypoxaemic and bradycardic events led to unintended hyperoxaemia (SpO2>95%).7 Both hypoxaemia and hyperoxaemia are associated with morbidity (impaired growth, bronchopulmonary dysplasia, retinopathy of prematurity, cerebral injury) and mortality. Reducing periods of hypoxaemia and hyperoxaemia may improve survival and neurodevelopmental outcome.6 ,8–13
Compliance in SpO2 targeting can be improved by training and implementation of guidelines.14–17 Additionally, FiO2 can be titrated automatically.18 ,19 Randomised trials comparing automated FiO2 systems with manual titration for short periods demonstrated an increase in the proportion of time spent with SpO2 within target range varying between 8% and 24%.20–23 Automated FiO2 control also decreased the required nursing time in preterm infants with frequent severe desaturations.20 ,24 ,25 However, the use of automated FiO2 control for longer periods has not been investigated.
In the neonatal intensive care unit (NICU) in Leiden University Medical Center (LUMC), an automated FiO2 control system (Closed Loop of inspired Oxygen, Avea-CLiO2, CareFusion, Yorba Linda, California, USA) was implemented and routinely used since August 2015 in order to improve the SpO2 targeting. We performed a retrospective study in preterm infants to evaluate automated FiO2 control when it was used as standard care and thus for a longer period. The aim was to compare the effectiveness of the automated FiO2 system versus manual titration of FiO2 in maintaining the SpO2 within the intended target range.
Methods
A prospective observational study was performed in the NICU of the LUMC, which is a tertiary-level perinatal centre in the Netherlands with an average of 650 intensive care admissions per year. In the Netherlands, no ethical approval is required for anonymised studies with medical charts and patient data that were collected and noted for standard care. The LUMC Medical Ethics Committee provided a statement of no objection for obtaining and publishing the anonymised data. All preterm infants <30 weeks of gestation (GA) admitted to the NICU before and after the implementation of the automated FiO2 control in August 2015 (May 2015–January 2016) receiving respiratory support (endotracheal and non-invasive ventilation) using the AVEA ventilator (CareFusion) were included. Preterm infants with major congenital heart disease were excluded.
The characteristics of each infant as well as clinical parameters and ventilator settings (including FiO2 and SpO2) were sampled every minute and routinely collected in the patient data management system (Metavision; IMDsoft, Tel Aviv, Israel). During both periods, the heart rate and SpO2 was collected using a neonatal pulse oximeter (Masimo Radical, Masimo Corporation, Irvine, California, USA) with an averaging time set at 8 s, integrated into the AVEA ventilator. Data were collected when infants received respiratory support by the AVEA and only included for analysis when supplemental oxygen was given, until the infants reached a GA of 32 weeks. After 32 weeks, most infants are transferred out of the intensive care area in our unit or to a regional hospital, where no automated FiO2 control is available.
During the manual and the automated FiO2 control periods, SpO2 was measured using a neonatal pulse oximeter integrated into the AVEA ventilator. During the manual period, the nurses manually titrated the supplemental oxygen following local guidelines. During the automated period, an automated FiO2 control device integrated in the ventilator was used (CLiO2), in addition to manual adjustments. The CLiO2 function is a closed-loop controller designed to regulate FiO2 levels for preterm infants receiving support and oxygen from a mechanical ventilator. The FiO2 is automatically adjusted to maintain the SpO2 within the target range set by the clinician.26 The CLiO2 was turned off during episodes where SpO2 remained 100% for more than 30 min when FiO2 was 0.21 as recurrent alarms would occur. In case extra oxygen was needed again, the CLiO2 was switched on, and data analysis continued. The episodes without extra oxygen were not included in the analysis. In this study, for both manual and automated FiO2 control periods, the SpO2 target range was 90–95% during oxygen therapy. The alarm was activated if SpO2 was below 90% or above 95%.
Before the start of each shift, the set target range of the CliO2 and alarm settings were checked by the nurse. Also back-up FiO2 was checked before the start of each shift or when a procedure was performed (eg, surfactant administration) and was adjusted if necessary. High FiO2 alarm was set at 70% with a delay of 60 s. All preterm infants received, as part of standard care, a loading dose of 10 mg/kg caffeine followed by 5 mg/kg/day. Dopram (2 mg/kg/hour) was added in case of refractory apnoeas.
The primary outcome was the percentage of time spent with SpO2 within the intended target range (90–95%) when FiO2 was >0.21. Also, the percentages of time spent with SpO2 >95%, >98%, <90%, <85% and <80% were calculated. Hypoxaemia was defined as SpO2 <80% and hyperoxaemia as SpO2 >95%.
Statistical analyses
Quantitative data are presented as median (IQR), mean (SD) or number (percentage) as appropriate. Time with SpO2 within various ranges for FiO2 >21% was collated for each infant and aggregated as percentages of the recorded time (median and IQR). A Kruskal-Wallis rank-sum test was used to compare the percentage of time that SpO2 was within the target range of 90–95% between the manual period and the automated period. A χ2 test was used to analyse discrete variables. If one of the cells had an expected count of less than five, Fisher's exact test was used. Statistical analyses were performed by IBM SPSS Statistics V.23 and R 3.2.0 (R Core Team (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/).
An increase of 10% of time that SpO2 was within the intended target range when using the automated FiO2 control was considered clinically relevant. On the basis of a previous study, we estimated an SD of 10%.27 Therefore, 21 patients in each arm were required to detect a change of 10% SpO2-wtr between the periods with an 80% power and a significance level of 0.05.
Results
In a 9-month period, 42 infants with a GA<30 weeks were admitted and supported using the AVEA ventilator, of which 21 infants <30 weeks in 4 months before the implementation of the automated FiO2 control and 21 infants in 5 months after implementation (characteristics table 1). In one patient, the CLiO2 was turned off for 3 days and during that period SpO2 data points were excluded from the analysis. In total, 234.541 data points (minute values) during the manual period and 392.211 data points (minute values) during the automated period were collected when FiO2 >21%. The median (IQR) number of data points per infant were not significantly different (manual vs automated period: 4805 (1238–16980) vs 16527 (1324–33625) data points; ns). The total number of days preterm infants were on respiratory support (with or without extra oxygen) was not different 16 (10–22) vs 14 (3–28) days; ns).
Patient characteristics, manual versus automated oxygen titration period
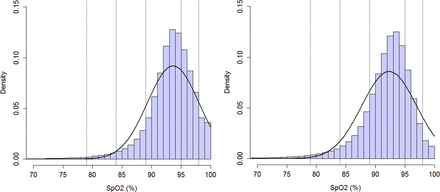
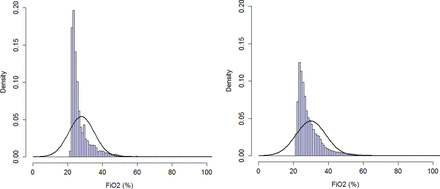
After implementation of the automated FiO2 control, there was a slight but significant decrease in median (IQR) SpO2 (manual vs automated: 94 (92–96)% vs 93 (91–95)%; p<0.001) (figure 1), while the FiO2 used increased (25 (24–29)% vs 27 (25–32)%; p<0.009) (figure 2). The time spent with SpO2 within target range increased during the automated period (48.4 (41.5–56.4)% vs 61.9 (48.5–72.3)%; p<0.01) (distribution is given in figure 1). The time spent with SpO2 >95% significantly decreased during the automated period (41.9 (30.6–49.4)% vs 19.3 (11.5–24.5)%; p<0.001) as did SpO2 >98% (10.1 (3.7–14.4)% vs 2.1(0.7–3.1)%, p<0.0005) (table 2). The time spent with SpO2<90% significantly increased during the automated period (8.6 (7.2–11.7)% vs 15.1 (14.0–21.1)%; p<0.0001), which was mostly influenced by an increase in time SpO2 was between 85% and 89% (table 2). There was no significant difference in time spent with SpO2<85% (2.7 (1.4–4.0)% vs 3.2 (1.8–5.1)%; ns), or % time with SpO2<80% (1.1 (0.4–1.7)% vs 0.9 (0.5–2.1)%; ns).
Time with SpO2 values within and outside the target range with FiO2>21%
Time with pulse oxygen saturation (SpO2) within various ranges collated over all infants and aggregated as total proportion of recorded time. Histogram on left side: manual period; histogram on right side: automated period. The smoothed bell-shaped line represents a fitted normal density function parameterised by the empirical mean and SD estimated from the proportion data of recorded time within various SpO2 ranges. The distribution of the proportional recorded time data is slightly negatively skewed with a long tail at the left and a higher mass at the right-hand side compared with a normal distribution.
Time with fraction of inspired oxygen (FiO2) within various ranges were collated over all infants and aggregated as total proportion of recorded time. Histogram on left side: manual period; histogram on right side: automated period.
Discussion
We observed that after implementing automated FiO2 control for routine care preterm infants spent significantly more time with SpO2 within their intended target range and less time with SpO2 above their intended target range, while FiO2 used was higher. Although the infants spent more time with SpO2 between 80% and 90% during automated FiO2 control, no significant effect on the time spent with hypoxaemia (SpO2<80%) was observed. It is likely that automatic FiO2 control had little effect on the infants' intrinsic stability, but rather that correction of fluctuations in SpO2 were faster than during manual oxygen titration and with less overshoot. Further, the use of an automated device precludes the tendency of nurses to maintain the SpO2 in the higher end of the target range, which could lead to more hyperoxaemia.1–5 The effect of an increased time that SpO2 was between 80% and 90% is unclear, while the reduction in hyperoxaemia may reduce the risk of major morbidities.6–13 ,26 Routine use of automated oxygen control has the potential to improve outcome in preterm infants.
Randomised and non-randomised studies have compared short periods using automated FiO2 with manual titration.9 ,20–23 ,26–28 This is the first study reporting the impact when an automated FiO2 was implemented in routine care for longer periods. Although the previous studies showed that automated FiO2 control improved time spent with SpO2 within the intended target range, the short study periods may have increased the risk for a Hawthorne effect.9 ,20–23 ,26–28 We compared automated FiO2 with manual titration for a much longer period and observed a bigger increase in time SpO2 was within the target range than has been observed in other studies. This is important as it supports that in routine use the potential for improvement of automated FiO2 is higher. This was not a randomised trial but our results reflect the effect of the automated FiO2 control when there was less risk that the attentiveness of caregivers was influenced by participating in a study. It is likely that the results of this study can be extrapolated to other level III NICU centres.
Whether there was a decrease in time SpO2 was above or below the target range or both varied between previous reported studies.9 ,20–23 ,26–28 We observed a decrease in time with SpO2 above target range, which was comparable with previous studies.9 ,20 ,22 ,23 ,26 ,28 While some studies of automated FiO2 control observed a decrease in time spent with SpO2 below target ranges,21 ,22 ,27 ,28 we observed an increase. This has also been reported by others.20 ,26 Explaining this conflicting finding is complicated by differences in methodology used (devices, study period, target range).21 ,22 ,27 We observed the largest increase in time spent just below target range (85–90%) with no increase in hypoxaemia (<80%), consistent with others.20 ,26 The CliO2 algorithm has been designed to prevent hyperoxaemia when overshoot occurs when the oxygen is increased. It is also known that nurses tend to give more liberal oxygen during desaturation resulting in a shorter duration with SpO2 below target range, but longer duration with time above target range. Indeed, in a previous study we reported that there is more awareness for alarms for SpO2 below the target range than above.7
Comparable to most previous studies, we could not detect a decrease in the total time with hypoxaemia when automated FiO2 was implemented. This likely reflects the aversion of caregivers to very low SpO2 values.9 ,20 ,23 ,26 Apparently the occurrence and depth of hypoxaemia is not prevented, but infants profit from a faster response provided by an automated FiO2 device when an hypoxaemic event occurs. The observed small increase in average FiO2 given could reflect the gradual downward titration defined by the algorithm of the device. Likewise, the gradual but constant downward titration of oxygen of the automated FiO2 control explains of the decrease in hyperoxaemia. It is possible that other devices for auto FiO2 control give different results as the algorithms can differ.29
In considering the results of our study and others, it is clear that the SpO2 distribution achieved using manual control differs from that achieved using automated control, even when the intended target range is the same.30 Others have also shown the effect of shifting automated control ranges.30 For that reason selecting the best target range for use with automated control should consider the likely SpO2 exposure and not just an adoption of the optimum standard of practice for manual control.
This study was performed as an audit after implementation of automated FiO2 control as standard care in our unit. The results reflect the real situation as data were collected for the duration infants were admitted, while nurses taking care of them and where workload varied. Although the characteristics of the groups were similar, this was not a randomised study and it is possible that there were differences between the groups of infants admitted during the observed periods. We compared SpO2 values that were routinely sampled every minute and because the value is an average of 8 s it is possible we missed SpO2 fluctuations in between the samples taken.31 However, our findings and distribution of SpO2 in the compared groups are similar when higher sample rates were used32 ,33 and it is likely that this is an accurate reflection of the SpO2 of the infants admitted.
Reducing the occurrence and duration of hypoxaemia and hyperoxaemia is known to reduce the related morbidity and mortality. Currently randomised trials are planned to determine the effect of automated FiO2 on clinical outcome in preterm infants.34 In anticipation of these upcoming trials, we implemented the automated FiO2 as standard care for all infants receiving respiratory support in the NICU as part of a quality improvement in our unit. Although difficult to measure, during evaluations nurses reported that after implementation of the automated FiO2 control their workload was less and they would be very reluctant to go back to the manual titration. Studies have reported that automated FiO2 control decreased the required nursing time in preterm infants with frequent severe desaturations.20 ,24 ,25 However, thresholds should be set very carefully in order not to mask deterioration of a patient and nurses needs to stay attentive as well as the automated FiO2 control should give a warning if the FiO2 baseline rises above a predefined level.
In conclusion, implementation of automated FiO2 control led to an increased compliance of maintaining SpO2 within the intended target range during oxygen therapy, with a decrease in the time SpO2>95% and SpO2>98%. Although the observed effects of the automated FiO2 control have the potential to improve outcome, this study was not designed to demonstrate this. Randomised studies are needed to confirm the beneficial effects of the automated FiO2 control on the outcome of preterm infants.
References
Footnotes
Twitter Follow Henriëtte van Zanten @heza01
Contributors HAVZ was the executive researcher of the study. She performed literature search, data collection, data analysis, data interpretation, writing and submitting of the manuscript. KLAMK was involved in data collection, critically reviewed the manuscript and approved the final version. BJS was involved in interpretation of the data, critically reviewed the manuscript and approved the final version. TB critically reviewed the manuscript and approved the final version. SP was involved in data analysis, critically reviewed the manuscript and approved the final version. ABtP was the project leader and performed literature search, designed the study and coordinated data analysis, data interpretation, writing, editing and submitting of the manuscript.
Competing interests None declared.
Ethics approval the Research Ethics Committee of LUMC.
Provenance and peer review Not commissioned; externally peer reviewed.