Article Text
Abstract
AIMS To compare the effects of prone and supine sleep position on the main physiological responses to mild asphyxia: increase in ventilation and arousal.
METHODS Ventilatory and arousal responses to mild asphyxia (hypercapnia/hypoxia) were measured in 53 healthy infants at newborn and 3 months of age, during quiet sleep (QS) and active sleep (AS), and in supine and prone sleep positions. The asphyxial test mimicked face down rebreathing by slowly altering the inspired air: CO2, maximum 5% and O2, minimum 13.5%. The change in ventilation with inspired CO2 was measured over 5–6 minutes of the test. The slope of a linear curve fit relating inspired CO2 to the logarithm of ventilation was taken as a quantitative measure of ventilatory asphyxial sensitivity (VAS). Sleep state and arousal were determined by behavioural criteria.
RESULTS At 3 months of age, prone positioning in AS lowered VAS (0.184 prone v0.269 supine, p = 0.050). At newborn age, sleep position had no effect on VAS. Infants aged 3 months were twice as likely to arouse to the test than newborns (p = 0.013). Placing infants prone as opposed to supine increased the chances of arousal 1.57-fold (p = 0.035).
CONCLUSION Our findings show 3 month old babies sleeping prone compared to supine have poorer ventilatory responses to mild asphyxia, particularly in AS, but the increased prevalence of arousal is a protective factor.
- asphyxia
- prone position
- supine position
- sudden infant death syndrome
Statistics from Altmetric.com
Prone sleeping has proved to be the main modifiable risk factor for sudden infant death syndrome.1-3 Several mechanisms have been proposed to explain the association. One of these is that infants may suffocate when breathing face down into soft bedding as a result of rebreathing their own expired gas and thus become exposed to the combined asphyxial stimulus of hypoxia and hypercapnia.4 ,5 The prone sleep position as compared to supine enhances sleep and is associated with fewer arousals from both spontaneous and external stimuli.6 ,7 The effect of sleep position on physiological responses to respiratory stimuli such as rebreathing is unknown.
Prone positioning in healthy preterm and full term infants makes spontaneous breathing easier. Beyond the neonatal period this mechanical advantage is not apparent for the healthy infant and adverse effects of prone sleeping on ventilation and oxygenation have been reported.8 ,9 The effect of sleep position on ventilatory responses to respiratory stimuli has been documented only in healthy preterm infants, in whom responses to CO2 were enhanced prone compared to supine.10 As far as we are aware, sleep position effects on the ventilatory responses to respiratory stimuli of infants at full term or beyond has never been studied.
The purpose of the present study was to compare ventilatory and arousal responses to mild asphyxia in infants sleeping prone and supine and to document the changes with sleep state and age. Our method used a hood to deliver gases slowly so that the build up of CO2 and depletion of O2 was similar to that which an infant would inspire during sleep face down on soft bedding (maximum 5% CO2; minimum 13.5% O2 over 5–6 minutes). Gas concentrations and delivery rates were designed to mimic the time course of rebreathing as shown using a mechanical model.5These compare well with those measured directly from a baby breathing face down into soft bedding (6.4% over 5.5 minutes).11
Methods
STUDY GROUP
A total of 53 babies (30 girls, 23 boys) were recruited for the study. Of these, 42 (80%) were studied at newborn age (15–28 days) and again at approximately 3 months of age (77–99 days). A further seven were studied at the newborn age only and four at the 3 month age only. Mean weight at newborn age was 3919 g (range 3040 to 4880 g) and at 3 months, 5715 g (range 4670 to 7180 g). All babies were born at Queen Mary Maternity Unit, Dunedin Hospital. Recruitment was by personal contact to mothers whose medical records fitted within our inclusion and exclusion criteria. Exclusion criteria were: living outside the Dunedin area, postnatal illness, major congenital abnormality, or illness at the time of study. Informed written consent was obtained from the parent(s) of all infants. Mothers of all infants were non-smokers and the majority of babies were breast fed at both study ages. One infant routinely slept prone whereas the others slept on their side or back. Each newborn study was completed in one day, whereas the majority of 3 month studies were completed over two days (at least five days apart). The study was approved by the Ethics Committee Otago, Southern Regional Health Authority, Dunedin, New Zealand.
STUDY PROTOCOL
Infants were studied during a morning or afternoon sleep in a quiet research nursery located within Dunedin Public Hospital. The infants were set up for recordings and then fed and positioned on a firm mattress. The first sleep position was determined randomly (not necessarily the preferred position) as either prone or supine and the same baby was then studied later in the opposite sleep position.
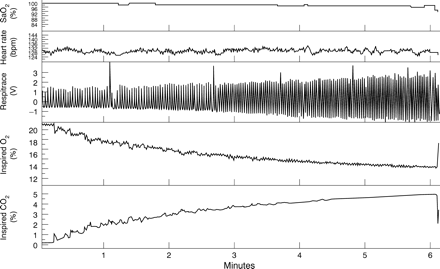
Once the infant was asleep, a perspex head box (suitable size for each age) was positioned over the head. Air first flowed through the head box from a Douglas bag at a rate of 6 l/min (newborn) or 9 l/min (3 month) for one minute and then the flow was switched over to the test mix bag containing 5.5% CO2 and 13% O2 in N2 which mixed with the contents of the head box to progressively alter the inspired gas mixture as shown in fig 1. The test mix was delivered for 5–6 minutes maximum or terminated earlier if the infant woke before this. The maximum inspired CO2and minimum inspired O2 reached were approximately 5% and 13.5% respectively. The gas sample probe was suspended within the head box and repositioned with change in sleep position so that it was always 4 cm directly above the level of the nostril. If possible tests were performed twice in each sleep state and sleep position. If sleep state changed during a test, the test was abandoned. No more than three tests were performed over one hour; 10 minutes was the minimum time between tests. The head positioning was checked so as to avoid spontaneous rebreathing; the majority of infants slept naturally with their face to the side but a few of the 3 month olds who wanted to sleep with their face into the mattress were positioned with their face to the side.
Typical waveforms from an asphyxial test. The subject was a 3 month old infant in quiet sleep.
RECORDINGS
The respiratory pattern was measured by inductive plethysmography (Respitrace model 150; Respitrace Co., New York, USA), a technique proposed by Konno and Mead,12 which considers that the respiratory system moves with two degrees of freedom of motion; the sum of the displacement detected by bands placed around the ribcage and abdomen equals tidal volume measured at the mouth. In studies of infants and children, there is good correlation between Respitrace sum and tidal volume so long as the studies are short term and in the controlled laboratory setting.10 ,13 ,14
Calibration of the Respitrace is position dependent,15 and as we were carrying out our ventilatory tests in two opposing sleep positions, only changes in uncalibrated tidal volume (voltage) and respiratory rate (breaths/min) from baseline were measured. The sum of the two signals analogous to the cross sectional area of the ribcage and abdomen were weighted routinely with the ratio 10:8. This is not a critical figure if the two compartments are in phase with each other.16 If, however, there was some “paradoxical movement”, when for part or all of the respiratory cycle the compartments were in opposite phase, this was no longer true, and tidal volumes could not be estimated with confidence. To avoid subjective decisions we compared computed sums of the individual signals, using several weightings, with the standard sum signal. If there were significant differences in the resultant estimates of tidal volume changes then the data were discarded as unreliable.
Heart rate and arterial oxygen saturation (Sao 2) were measured from the pulse oximeter (Nellcor N-200, Nellcor Hayward, California, USA) with averaging time set to three seconds. The percentage of inspired O2 and CO2 was measured from a Datex gas analyser (Normocorp 200-oxy CO2-O2, Datex Instumentarium Corp., Helsinki, Finland). The signals from the pulse oximeter, Respitrace, and Datex were relayed through the integrated hardware/software system of the MacLab (ADInstruments Pty Ltd, Australia). Sleep state was determined by watching eye, mouth, hand, and respiratory trace movements based on those described for the two age groups by Guilleminault and Souquet.17 Generally quiet sleep (QS) contained regular breathing with no rapid eye movements or facial movements. Active sleep (AS) contained some rapid eye movements, irregular breathing, and sometimes hand or mouth movements. Arousal was evident when the infant's eyes were open with vigorous movement and/or crying.
MEASUREMENT OF THE VENTILATORY ASPHYXIAL SENSITIVITY (VAS)
The change in ventilation (product of respiratory rate and tidal volume) associated with the percentage of inspired CO2 was measured every minute over the 5–6 minutes of the asphyxial test. A linear curve fit relating inspired CO2 to the natural logarithm of ventilation as described by Campbell and colleagues18 was then used to determine the ventilatory asphyxial sensitivity (VAS). The value of VAS was the slope of that line and was a measure of the sensitivity of the ventilatory system to the asphyxial test.
STATISTICAL ANALYSES
For every infant a mean baseline value for each asphyxial test was obtained for Sao2, respiratory frequency, tidal volume, and heart rate over a period of 10 breaths immediately before delivery of the test gas. Mean values for the same variables were then taken over the 10 breaths immediately before the test was stopped (final values). A single value for VAS was obtained for each test. As the data from these babies involves analysis of many interacting factors, a multivariate analysis isolating the effect of each factor in turn was adopted. This procedure is described in the manual for the statistical package used (STATA, Stata Statistical Software: Release 6.0, Stata Corporation, College Station, Texas, USA).19All the observations and interactions were considered for inclusion in the model for regression analysis. This means that the standard errors and the significance of the statistical tests were based on the number of babies rather than the number of observations in the study. Main effects were analysed for age, sleep state, and sleep position and because the interaction effect between these factors was statistically significant for position, its effect was estimated within each age level and sleep state. Logistic regression was used to compare arousal data for the three variables. Differences were considered statistically significant at p ⩽ 0.05.
Results
Figure 1 shows a typical example of an asphyxial test and recorded variables. Table 1 presents data for baseline Sao2, heart rate, and respiratory rate. The data are presented as the changes in stated variables with change of individual “factors”, age, sleep state, or position. Baseline Sao 2 increased significantly with age, was lower in AS compared to QS, and lower prone compared to supine (p = 0.007); significant at newborn age only. Heart rate decreased with age, was higher prone, and higher in AS. There was a significant interaction effect of sleep position with age (p = 0.004); the higher heart rate prone was significantly greater at 3 months of age. Overall baseline respiratory rate decreased significantly with age, was higher in AS than QS, but was less prone compared to supine. There was a significant interaction effect of sleep position with age; the lower respiratory rate prone compared to supine at 1 month (−5.4 breaths/min) was not significant by 3 months of age (−0.8 breaths/min).
Baseline values: the effects of age, sleep position, and sleep state on Sao 2, heart rate, and respiratory rate
At the end of the asphyxial test, all final values were significantly different from baseline (p < 0.0001). Mean estimates for the changes were as follows: Sao 2, −0.9% (CI −1.1, −0.7); heart rate, + 6.7 beats/min (CI 5.8, 7.5); respiratory rate, +5.7 breaths/min (CI 3.6, 6.4). Tests that terminated in arousal showed no difference in these variables compared with non-arousal tests. By the end of the test, final Sao 2 values were similar for age but still significantly lower in AS and in the prone position (table 2). Heart rate and respiratory rate were now unaffected by sleep position. Mean final values of inspired CO2 and O2 were 4.84% (CI 4.82, 4.86) and 13.99% (CI 13.95, 14.03) respectively when tests resulted in no arousal. When arousal occurred, the test was terminated early and the inspired gas levels were therefore less changed from room air (0.60% less for CO2 and 1.0% more for O2; p < 0.0001). Arousals were more common in older babies and in AS sleep (see below).
Final values: data comparing the effects of age, sleep position, and sleep state on Sao 2, heart rate, and respiratory rate
VAS is the slope of inspired CO2 plotted against the natural logarithm of ventilation; the mean correlation coefficients were 0.85 for QS and 0.67 for AS. Table 3 presents mean values of VAS by age, sleep state, and sleep position together with a statistical summary. At newborn age, sleep position had no significant effect on VAS (in either QS or AS). However at 3 months of age VAS was significantly lower when infants were sleeping prone compared to supine, but only in AS.
VAS: mean (SEM) values presented by age, sleep state, and sleep position and statistical summary
Table 4 gives the incidence of arousal from the asphyxial test. Age, sleep state, and sleep position all caused significant changes. Infants aged 3 months were twice as likely to arouse to the test than newborn infants. The test given in AS compared to QS increased the chances of arousal fivefold and placing infants prone as opposed to supine increased the chances of arousal 1.57-fold. There were no interaction effects.
Prevalence of arousal to the asphyxial test
Discussion
The main finding of this study was that prone positioning of 3 month old infants resulted in a reduction in ventilatory sensitivity to the asphyxial test compared to supine positioning and this effect occurred during active sleep only. The findings add support to the evidence that prone positioning, at the age at which infants are at greatest risk of sudden infant death syndrome (SIDS), can depress or adversely effect normal physiological responses.7 ,20 ,21In our study, at newborn age, sleep position had no effect on ventilatory responses.
The asphyxial challenge applied in active sleep, irrespective of position and age, always resulted in a decreased ventilatory response compared with quiet sleep, supporting the findings of others.18 ,22 ,23 This decreased response is thought to occur because of the loss of intercostal muscle tone resulting in a decrease in the ribcage contribution to ventilation during CO2 testing,22 inhibition of abdominal muscle recruitment,23 and a decrease in central output to the diaphragm.24 In considering why the response should be further attenuated in 3 month old infants sleeping prone compared to supine and not in newborns, we speculate that weight of the heavier 3 month old could be an additional factor imposing a mechanical limitation to effective lung expansion. The greater force required to expand the thoracic cage could explain the attenuated ventilatory response. Although this effect of extra weight would also be a factor in infants sleeping prone in quiet sleep, maintenance of intercostal tone may have ensured effective lung expansion. Our own observations were that the newborn infant tended to sleep prone with knees tucked up, leaving a gap between abdomen and mattress, whereas the 3 month old infant tended not to tuck his/her knees up and the much larger abdomen was compressed against the mattress. In the adult, the prone position can alter respiratory mechanics, decreasing compliance as the abdomen is compressed, and restricting chest wall movement.25 ,26
Prone positioning generally improves lung function and respiratory drive in preterm and full term newborn infants with or without respiratory illness.27 ,28 Beyond the newborn period there is no advantage to ventilation in healthy infants sleeping prone.6 ,29 Our data support this in that respiratory rate decreased at newborn age with prone positioning suggesting improved ventilation, but at 3 months of age, changing position made no difference. Martin et al, in a study of healthy preterm infants, found that the ventilatory response to hypercapnia alone in healthy preterm infants was enhanced with prone positioning.10 This is opposite to what we found at 3 months of age with hypercapnia/hypoxia. It is difficult to compare the two studies given the disparate ages and differences in methodology; what is consistent is that both studies show an advantage to ventilation in young infants sleeping prone. As the infants get older and reach the age that they are at highest risk of SIDS (3 months), the present study goes on to expose a disadvantage to ventilation with prone sleeping.
Our finding of higher baseline heart rate prone compared to supine has been previously reported in a study in which higher skin temperatures were also measured.30 This links with the finding that sleeping prone reduces the exposed surface area available for heat exchange and increases metabolic rate.31 ,32 Our reported changes in heart rate, though consistent with this, were minimal.
Arousal, most often associated with lifting and/or turning of the head, is seen as a primary defence against asphyxia—that is, if in a real situation, the baby was breathing face down into soft bedding, lifting and/or turning of the head would allow the baby to breathe fresh air and waking would allow conscious control of a movement and crying if necessary. We acknowledge that the airway protective measure of head lifting or turning alone can occur before full arousal,33but this alone was not an appropriate end point for us. The prone position compared to supine restricted head movement involving lifting, particularly in the younger infants. Therefore the criteria of eyes open and or crying was chosen as the end point for arousal suitable to compare both sleep positions. Even full arousal may not be enough of a defence if the baby was under the covers of the bedclothes or unable to move away from the asphyxiating surface, for example, a hollow in a polystyrene pillow.4
The general consensus is that prone positioning enhances sleep, reducing movement30 and arousal, either spontaneous6 ,30 or provoked.20 Our previous work supports this consensus in that 3 month old infants sleeping prone compared to supine were less likely to arouse in active sleep to a head up tilt test.7 However in the present study we found the opposite effect of sleep position on arousal—that is, at 3 months of age and in active sleep, infants aroused more readily to the asphyxial test in prone sleep compared to supine, probably because of the attenuated ventilatory response allowing more change in blood gases. The prone position in the heavier 3 month old baby with the low intercostal muscle tone of active sleep results in a greater inspiratory effort to achieve normal response from lung mechanoreceptors. This is a recognised cause of dyspnoea34and presumably of arousal. In quiet sleep when ventilatory responses were good, arousal from the asphyxial test was much less common, irrespective of age or sleep position.
The fact that the infants aroused more readily in active sleep is reassuring for the infants studied here who were at very low risk of SIDS. However, babies who have died of SIDS have neuropathological findings suggestive of delayed development in areas of the brainstem responsible for cardiorespiratory control and arousal.35 A vulnerable infant who at a critical time in development fails to respond and arouse to an exogenous stressor such as those associated with the prone position could go on to die of SIDS as proposed by Filiano and Kinney.36
O2 saturation levels never became clinically low nor were there any heart rate changes of clinical significance, emphasising the safety of our protocol and the mild nature of the asphyxial stimulus. The increase in ventilation associated with the hypercapnic stimulus minimised the fall in alveolar oxygen levels, thus preventing any significant fall in oxygen saturation. With the combination of factors resulting in a decreased ventilatory response to the asphyxial test (3 month, prone, and in active sleep), 61.2% of the tests resulted in no arousal. We do not know what would have happened had the test been able to continue beyond the limits of our protocol. If an infant still did not wake, failure to mount an appropriate ventilatory response could have fatal consequences. Chiodini and Thach11 have studied infants naturally sleeping face down into soft bedding. One infant reached 85% saturation in response to accumulation of 6.4% CO2 with a Paco 2 of 66 mm Hg, at which time they moved the infant to the face to side position. Other studies testing hypoxia and hypercapnia report infants with poor ventilatory responses16 ,37 and/or suppressed arousal.38 ,39
Currently the prone sleep position is without doubt the major risk factor for SIDS. We speculate from our findings that infants sleeping face down who do not mount an appropriate ventilatory response to asphyxia and who do not arouse, may go on to die of SIDS. This would be most likely to occur in infants greater than 1 month of age and occur in active sleep.
Acknowledgments
This work was supported by a grant from the Health Research Council of New Zealand. We are grateful to the families whose infants were part of this study.