Abstract
Objective:
To describe the current use of treatments to prevent or treat patent ductus arteriosus (PDA) in preterm infants, examine the association between different treatment strategies and neonatal outcomes and review the variation in these practices between centers.
Study design:
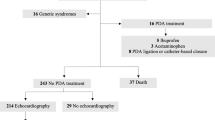
Cohort study of infants born between 23 and 30 weeks gestation managed by the Pediatrix Medical Group from 1997 to 2004. We collected data on demographics, indomethacin and ligation, and outcomes of the following five groups: prophylactic indomethacin treatment: infants treated with indomethacin on day of life (DOL) 0 or 1; indicated indomethacin treatment: infants treated with indomethacin after DOL 1; PDA without treatment: infants with a PDA without report of treatment; ligation only: infants with a PDA ligation without use of indomethacin and no PDA: infants without a PDA and without treatment.
Results:
There were 6189 (18%) patients who received prophylactic indomethacin, 5690 (16%) patients received indicated treatment, 3886 (11%) patients had a PDA without treatment, 702 (2%) patients received ligation only and 18 136 (52%) patients had no PDA. In multivariate analysis, mortality among survivors to 2 days of age was lower (odds ratio (OR) 0.6, 95% confidence interval (CI) 0.5 to 0.7, P<0.01) and chronic lung disease, isolated intestinal perforation and severe retinopathy of prematurity (stages 3 and 4) were higher (OR 1.5, 95% CI 1.3 to 1.6, P<0.01; OR 1.5, 95% CI 1.1 to 2.0, P<0.01 and 1.4, 95% CI 1.2 to 1.6, P<0.01, respectively) in the indicated treatment group compared with the PDA without treatment group. The proportion of infants receiving prophylactic indomethacin among all infants and infants receiving indicated treatment among neonates with a report of a PDA varied by site from 0 to 59% (median 9.5%) and 0 to 100% (median 62%), respectively.
Conclusions:
Indomethacin use for intraventricular hemorrhage prevention and/or treatment of a PDA is common, but the selection of infants for treatment, and the decision of when and how to treat vary widely between centers. Our findings suggest the need for randomized, placebo-controlled trials of the effect of treatment of the PDA in preterm infants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dollberg S, Lusky A, Reichman B . Patent ductus arteriosus, indomethacin and necrotizing enterocolitis in very low birth weight infants: a population-based study. J Pediatr Gastroenterol Nutr 2005; 40 (2): 184–188.
Marshall DD, Kotelchuck M, Young TE, Bose CL, Kruyer L, O’Shea TM . Risk factors for chronic lung disease in the surfactant era: a North Carolina population-based study of very low birth weight infants. North Carolina Neonatologists Association Pediatrics 1999; 104 (6): 1345–1350.
Rojas MA, Gonzalez A, Bancalari E, Claure N, Poole C, Silva-Neto G . Changing trends in the epidemiology and pathogenesis of neonatal chronic lung disease. J Pediatr 1995; 126 (4): 605–610.
Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S et al. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med 2001; 344 (26): 1966–1972.
Shorter NA, Liu JY, Mooney DP, Harmon BJ . Indomethacin-associated bowel perforations: a study of possible risk factors. J Pediatr Surg 1999; 34 (3): 442–444.
Stark AR, Carlo WA, Tyson JE, Papile LA, Wright LL, Shankaran S et al. Adverse effects of early dexamethasone in extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med 2001; 344 (2): 95–101.
Watterberg KL, Gerdes JS, Cole CH, Aucott SW, Thilo EH, Mammel MC et al. Prophylaxis of early adrenal insufficiency to prevent bronchopulmonary dysplasia: a multicenter trial. Pediatrics 2004; 114 (6): 1649–1657.
Zbar RI, Chen AH, Behrendt DM, Bell EF, Smith RJ . Incidence of vocal fold paralysis in infants undergoing ligation of patent ductus arteriosus. Ann Thorac Surg 1996; 61 (3): 814–816.
Laughon MM, Simmons MA, Bose CL . Patency of the ductus arteriosus in the premature infant: is it pathologic? Should it be treated? Curr Opin Pediatr 2004; 16 (2): 146–151.
Thorp JA, Jones PG, Knox E, Clark RH . Does antenatal corticosteroid therapy affect birth weight and head circumference? Obstet Gynecol 2002; 99 (1): 101–108.
Clark RH, Bloom BT, Spitzer AR, Gerstmann DR . Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics 2006; 117 (1): 67–74.
Clark RH, Bloom BT, Spitzer AR, Gerstmann DR . Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics 2006; 117 (6): 1979–1987.
Narayanan M, Cooper B, Weiss H, Clyman RI . Prophylactic indomethacin: factors determining permanent ductus arteriosus closure. J Pediatr 2000; 136 (3): 330–337.
Attridge JT, Clark R, Walker MW, Gordon PV . New insights into spontaneous intestinal perforation using a national data set: (1) SIP is associated with early indomethacin exposure. J Perinatol 2006; 26 (2): 93–99.
Author information
Authors and Affiliations
Corresponding author
Additional information
There is no financial disclosure for the authors to report.
Rights and permissions
About this article
Cite this article
Laughon, M., Bose, C. & Clark, R. Treatment strategies to prevent or close a patent ductus arteriosus in preterm infants and outcomes. J Perinatol 27, 164–170 (2007). https://doi.org/10.1038/sj.jp.7211662
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jp.7211662
Keywords
This article is cited by
-
Ductus Arteriosus of Extremely Preterm Twins is More Resistant to Cyclooxygenase Inhibitors Than Those of Singletons
Pediatric Cardiology (2022)
-
Restrictive Threshold for the Management of Patent Ductus Arteriosus in Very Low Birth Weight Neonates
Indian Pediatrics (2021)
-
Decrease in the frequency of treatment for patent ductus arteriosus after implementation of consensus guidelines: a 15-year experience
Journal of Perinatology (2019)
-
Early treatment versus expectative management of patent ductus arteriosus in preterm infants: a multicentre, randomised, non-inferiority trial in Europe (BeNeDuctus trial)
BMC Pediatrics (2018)
-
Near-infrared spectroscopy for detection of a significant patent ductus arteriosus
Pediatric Research (2016)