Abstract
Objective:
To determine (a) the proportion of asymptomatic infants born at ⩾35 weeks gestation evaluated for early-onset sepsis (EOS) and exposed to postnatal antibiotics; (b) reasons for and outcomes of the evaluations, and (c) anticipated changes when applying the Centers for Disease Control and Prevention (CDC) 2010 guidelines to this study population.
Study Design:
Retrospective cohort study of infants born at ⩾35 weeks gestation in 2008–2009 in a large maternity center.
Result:
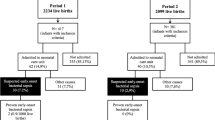
Out of the 7226 infants that met the study criteria: 1062 (14.7%) were evaluated for EOS and half of those evaluated, received empiric antibiotics. 70.4% of evaluations were performed owing to maternal intrapartum fever, but 23% were prompted by inadequate Group B Streptococcus (GBS) prophylaxis alone. Three cases of blood culture-proven infection were identified.
Conclusion:
Improved approaches are needed to identify asymptomatic infants who are at risk for EOS to decrease unnecessary evaluations and antibiotic exposure. Transition to the 2010 CDC GBS guidelines may eliminate a quarter of EOS evaluations among these infants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Centers for Disease Control and Prevention (CDC). Early-onset and late-onset neonatal group B streptococcal disease—United States, 1996–2004. MMWR Morb Mortal Wkly Rep 2005; 54: 1205–1208.
Centers for Disease Control and Prevention (CDC). Trends in perinatal group B streptococcal disease - United States, 2000–2006. Centers for Disease Control and Prevention (CDC). MMWR Morb Mortal Wkly Rep 2009; 58: 109–112.
Weston EJ, Pondo T, Lewis MM, Martell-Cleary P, Morin C, Jewell B et al. The burden of invasive early-onset neonatal sepsis in the United States, 2005–2008. Pediatr Infect Dis J 2011; 30: 937–941.
Puopolo KM, Eichenwald EC . No change in the incidence of ampicillin-resistant, neonatal, early-onset sepsis over 18 years. Pediatrics 2010; 125: e1031–e1038.
Centers for Disease Control and Prevention (CDC). Prevention of perinatal group B streptococcal disease: a public health perspective. Centers for disease control and prevention. MMWR Recomm Rep 1996; 45: 1–24.
Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A . Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep 2002; 51: 1–22.
Verani JR, McGee L, Schrag SJ . Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep 2010; 59: 1–36.
Benirschke K . Routes and types of infection in the fetus and the newborn. Am J Dis Child 1960; 99: 714–721.
Alexander JM, McIntire DM, Leveno KJ . Chorioamnionitis and the prognosis for term infants. Obstet Gynecol 1999; 94: 274–278.
Escobar GJ, Li DK, Armstrong MA, Gardner MN, Folck BF, Verdi JE et al. Neonatal sepsis workups in infants >/=2000 grams at birth: a population-based study. Pediatrics 2000; 106: 256–263.
Benitz WE, Gould JB, Druzin ML . Risk factors for early-onset group B streptococcal sepsis: estimation of odds ratios by critical literature review. Pediatrics 1999; 103: e77.
Van Dyke MK, Phares CR, Lynfield R, Thomas AR, Arnold KE, Craig AS et al. Evaluation of universal antenatal screening for group B streptococcus. N Engl J Med 2009; 360: 2626–2636.
Cotten CM, McDonald S, Stoll B, Goldberg RN, Poole K, Benjamin Jr DK . The association of third-generation cephalosporin use and invasive candidiasis in extremely low birth-weight infants. Pediatrics 2006; 118: 717–722.
Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 2009; 123: 58–66.
Kuppala VS, Meinzen-Der J, Morrow AL, Schibler KR . Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr 2011; 159: 720–725.
Vanaja N, Alexander VN, Northrup V, Bizzarro MJ . Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr 2011; 159: 392–397.
Alm B, Erdes L, Möllborg P, Pettersson R, Norvenius SG, Aberg N et al. Neonatal antibiotic treatment is a risk factor for early wheezing. Pediatrics 2008; 121: 697–702.
Goksör E, Alm B, Thengilsdottir H, Pettersson R, Åberg N, Wennergren G . Preschool wheeze - impact of early fish introduction and neonatal antibiotics. Acta Paediatr 2011; 100: 1561–1566.
Bitner-Glindzicz M, Pembrey M, Duncan A, Heron J, Ring SM, Hall A et al. Prevalence of mitochondrial 1555A-->G mutation in European children. N Engl J Med 2009; 360: 640–642.
Murgas Torrazza R, Neu J . The developing intestinal microbiome and its relationship to health and disease in the neonate. J Perinatol 2011; 31 (Suppl 1): S29–S34.
Puopolo KM, Draper D, Wi S, Newman TB, Zupancic J, Lieberman E et al. Estimating the probability of neonatal early-onset infection on the basis of maternal risk factors. Pediatrics 2011; 128: e1155–e1163.
Polin RA . Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics 2012; 129: 1006–1015.
Puopolo KM, Madoff LC, Eichenwald EC . Early-onset group B streptococcal disease in the era of maternal screening. Pediatrics 2005; 115: 1240–1246.
Boyer KM, Gotoff SP . Prevention of early-onset neonatal group B streptococcal disease with selective intrapartum.chemoprophylaxis. N Engl J Med 1986; 314: 1665–1669.
Newman TB, Puopolo KM, Wi S, Draper D, Escobar GJ . Interpreting complete blood counts soon after birth in newborns at risk for sepsis. Pediatrics 2010; 126: 903–909.
Van Dyke MK, Phares CR, Lynfield R, Thomas AR, Arnold KE, Craig AS et al. Evaluation of universal antenatal screening for group B streptococcus. N Engl J Med 2009; 360: 2626–2636.
Lieberman E, Lang JM, Frigoletto Jr F, Richardson DK, Ringer SA, Cohen A . Epidural analgesia, intrapartum fever, and neonatal sepsis evaluation. Pediatrics 1997; 99: 415–419.
No authors listed. Massachusetts maternity data as reported by hospitals. Available at http://www.mass.gov/eohhs/docs/dhcfp/qc/archives/qc2/mat-procedures.pdf. Accessed on 1 December 2011.
Acknowledgements
We thank the Dr Ruth Tuomala and Dr Alison Cape for the obstetrical contributions to the study; the Centers for Clinical Excellence at Brigham and Women's Hospital for hospital demographic information; and Dr Stella Kourembanas, the Division of Newborn Medicine at Children's Hospital and the Scholars in Clinical Science Program for ongoing support of Dr Mukhopadhyay.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict to interest.
Rights and permissions
About this article
Cite this article
Mukhopadhyay, S., Eichenwald, E. & Puopolo, K. Neonatal early-onset sepsis evaluations among well-appearing infants: projected impact of changes in CDC GBS guidelines. J Perinatol 33, 198–205 (2013). https://doi.org/10.1038/jp.2012.96
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2012.96
Keywords
This article is cited by
-
Early life inflammation is associated with spinal cord excitability and nociceptive sensitivity in human infants
Nature Communications (2022)
-
Noninfectious influencers of early-onset sepsis biomarkers
Pediatric Research (2022)
-
Effects of early-life antibiotics on the developing infant gut microbiome and resistome: a randomized trial
Nature Communications (2022)
-
Brief comments on three existing approaches for managing neonates at risk of early-onset sepsis
Italian Journal of Pediatrics (2021)
-
Infants exposed to antibiotics after birth have altered recognition memory responses at one month of age
Pediatric Research (2021)