Short bowel syndrome (SBS) is a condition characterised by an increased intestinal transit time, leading to diarrhoea and malabsorption of nutrients and, potentially, growth retardation. The most frequent underlying diagnoses in neonates are necrotising enterocolitis, volvulus, intestinal atresia and gastroschisis(Reference Sigalet1, Reference Koffeman, van Gemert and George2).
Bowel adaptation starts shortly after bowel resection and may last 1–2 years, during which nutrient absorption is relatively inadequate(Reference Goulet, Ruemmele and Lacaille3).
Improved care has led to increased survival rates of infants with SBS, but little information is available on the long-term impact of infantile SBS on growth and physical development. Short stature has been reported(Reference Vanderhoof and Young4), as well as delayed onset of puberty(Reference Delemarre-van de Waal, van Coeverden and Engelbregt5). The latter, however, is generally associated with chronic malabsorption, and with growth delay and the pubertal growth spurt(Reference Pozo and Argente6). Chronic illness with malabsorption also has a negative effect on bone maturation, as documented in children with inflammatory bowel disease(Reference Boot, Bouquet and Krenning7, Reference Cowan, Warner and Dunstan8).
The aim of the present cross-sectional study was to evaluate the long-term effects of infantile SBS on growth, nutritional status, defecation pattern and food intake.
Methods
Population
Children with SBS were identified from the medical databases and charts of the hospital, as reported elsewhere(Reference Olieman, Tibboel and Penning9). All surviving children or adults with infantile SBS (aged ≤ 1 year) treated in their first year of life in the Erasmus Medical Center, Sophia Children's Hospital between January 1975 and January 2003 were asked to participate. Patients with psychomotor retardation due to additional anomalies were excluded, because most measurements cannot reliably be performed. Patients who were still parenteral nutrition (PN) dependent at the time of measurement are excluded in order to reduce the heterogeneity of the present study group.
Definition of short bowel syndrome
The definition of SBS used in the present study was the one formulated by the Dutch Committee on Intestinal Failure:
(1) greater than 70 % resection of the small bowel(Reference Koffeman, van Gemert and George2, Reference Teitelbaum, Drongowski and Spivak10);
(2) PN needed for longer than 42 d after bowel resection(Reference Wales, de Silva and Kim11–Reference Kaufman, Loseke and Lupo14);
(3) residual small bowel length, distal to the ligament of Treitz, (50 cm for a premature neonate (gestational age 27–36 weeks) and < 75 cm for a term neonate(Reference Touloukian and Smith15, Reference Heij, Meijers-IJsselstijn and Olieman16).
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Erasmus Medical Center Ethical Review Board. Subjects and parents received written information on the study design, and written informed consent from the parents for subjects younger than 18 years and separately from subjects older than 12 years of age were obtained.
Study design
In the present single-centre, cross-sectional study, all diagnostic measurements concerning subjects' growth, nutritional and dietary status were performed during a single outpatient visit in the period from November 2005 to August 2007. Measurements were taken by a dietitian (nutritional assessment and dietary intake), physician (general health examination) or nuclear laboratory technician (dual-energy X-ray absorptiometry (DEXA) scan).
Clinical characteristics during the first year of life
Demographic data such as date of birth, sex, underlying diagnosis leading to SBS, gestational age and birth weight were collected retrospectively. Surgical reports were searched for the presence of the ileocaecal valve and the remaining small bowel length, measured distally to the ligament of Treitz. Percentage of remaining small bowel length was calculated from predicted bowel length for gestational age(Reference Touloukian and Smith15). Number of operations in the first year of SBS was counted. The number of central venous catheter (re)placements, as a consequence of occlusion, thrombosis or sepsis, was also recorded. Length of stay and PN duration were derived from data for the entire follow-up period (>1 year) until October 2007.
Dates of start and end of minimal enteral feeding(Reference Olieman, Tibboel and Penning9, Reference Tyson and Kennedy17) and enteral nutrition were collected. Type of nutrition was classified as breastfeeding, polymeric or semi-elemental. The numbers of interruptions of enteral nutrition, necessitated by inadequate passage through the gastrointestinal tract, were counted.
Detailed information on growth and nutrition in the first year of life of these patients has been published elsewhere(Reference Olieman, Tibboel and Penning9).
Measurements
Subjects were asked not to eat or drink within 2 h before measurement and to refrain from strenuous exercise on this day.
Height and weight
Body weight was recorded to the nearest 0·1 kg using an electrical scale (Seca Alpha 770, Hamburg, Germany). Height was measured to the nearest 0·1 cm using a stadiometer (Stanley Mabo, London, UK). The height of the patients was measured in the outpatient clinic, if possible, or by their general practitioners. The target height (TH) of the subjects was calculated as ((father's height+mother's height ± 13)/2)+4·5 cm. TH range was defined as TH standard deviation score (SDS) (1·3 ± SDS). In adults, the BMI was calculated using weight (kg)/ height (m)2.
Skinfolds
Skinfold thickness in the biceps, triceps, subscapular and supra-iliac region was measured three times(Reference Gerver and de Bruin18) to the nearest 0·1 mm using a Harpenden calliper (John Bull, British Indicators Ltd, Burgess Hill, West Sussex, UK) on the non-dominant side of the body, and the mean value was calculated. Body fat percentage (%BF) was calculated from the sum of four skinfold measurements in children(Reference Deurenberg, Pieters and Hautvast19) and in adults(Reference Durnin and Womersley20) using group-specific equations. Skinfold measurement is a cost-effective and non-invasive nutritional assessment method with reasonable accuracy(Reference Lohman21).
Dual-energy X-ray absorptiometry
Total body DEXA was performed using a Lunar-Prodigy (GE Healthcare, Waukesha, WI, USA) scanner in order to determine bone mineral density (BMD, g/cm2) of the lumbar spine (ls) and total body (tb). Total body DEXA also measured bone mineral content (BMC, g) and lean body mass (LBM, g), with %BF given for total tissue mass. Many studies found DEXA to be a good reference method for nutritional assessment, due to its high correspondence with outcome of isotope dilution techniques(Reference Lohman21–Reference Uszko-Lencer, Bothmer and van Pol23).
The values of BMDls, BMDtb, BMC, LBM and %BF (measured with DEXA) of the children were compared to Dutch reference data, depending on age and sex(Reference van der Sluis, de Ridder and Boot24), and expressed in SDS. Adult values of BMDls and BMDtb were compared to reference values delivered by the manufacturer and expressed in SDS.
Furthermore, measurements of skinfolds were compared to measurements of DEXA in children to examine the inter-relationship.
Dietary intake
Before the outpatient visit, subjects were asked to record quantities of foods and beverages consumed during a weekend day and on two week days. During the outpatient visit, a trained dietitian cross-checked the records and asked the subjects to specify entries, if necessary, and add missing items or amounts. The dietary intakes were compared to the recommended daily allowances for children and adults, and when appropriate, to the estimated average requirements, depending on age and sex(25, 26).
Defecation pattern
Defecation pattern was determined by a self-developed questionnaire based on the symptom checklist of Poley et al. (Reference Poley, Stolk and Tibboel27). It comprised stool frequency, self-estimated quantity of stool of the subjects, the Bristol stool form scale(Reference Heaton, Radvan and Cripps28) and applicable symptoms from the Rome II criteria(Reference Caplan, Walker and Rasquin29), such as bowel cramps, flatulence and bloating. As control data were not available, the questionnaire was additionally filled out by age- and sex-matched healthy controls, recruited through schools and the university in Rotterdam.
Current health status and Tanner stages
General medical, neurological and pubertal development(Reference Tanner30) was examined by a physician. For patients in puberty, delay in puberty was determined by comparing Tanner stage and age with reference data of Dutch children(Reference Roede and Van Wieringen31). Blood pressure (expressed in mmHg) and heart rate (beats per min) were measured once by Dinamap Procare (GE Healthcare). Values were compared to the reference data(32).
Statistical analysis
Group size was not based on a formal power analysis. The incidence of SBS in the Netherlands is unknown, but from clinical experience, it is judged to be relatively low. We therefore aimed at including all patients with SBS admitted to our hospital between 1975 and 2003. Descriptive statistics (frequencies, mean, median, standard deviations and range) were calculated. The patients were stratified in two age groups, namely, 5–18 years (children) and over 18-year-old patients (adults), and differentiated by sex.
Kruskal–Wallis tests and χ2 tests served to identify differences between the study group and all eligible patients. Cystic fibrosis itself might be associated with impaired growth; therefore, data for the whole study group were compared by appropriate tests to data for a subgroup excluding patients with cystic fibrosis.
Values of weight, height, TH and skinfolds were compared to national standards(Reference Fredriks, van Buuren and Burgmeijer33–Reference Gerver and de Bruin36) and expressed in SDS, depending on sex, age and race (Growth Analyser version 3; Dutch Growth Foundation, Rotterdam, the Netherlands).
Bland–Altman plots were used to assess the agreement between outcomes of skinfold measurements and DEXA(Reference Bland and Altman37). One-sample t tests were performed to compare the mean SDS values with normal values. Means were compared using paired t tests. When data were not normally distributed, median values were compared using the Wilcoxon rank test or χ2 test. The level of significance was set at 0·05.
Results
Of the seventy-two eligible subjects, thirty-two did not participate in the present study because either they did not give informed consent (n 15) or they could not be located (n 17; Fig. 1). Kruskal–Wallis tests and χ2 tests identified no differences in underlying diagnoses, sex, age, percentages of premature birth or length of remaining bowel between the groups ‘included’, ‘no informed consent’ and ‘not located’ (data not shown).
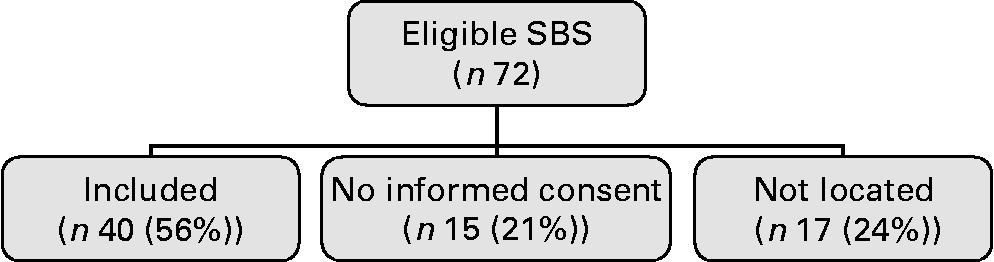
Fig. 1 Flow chart study. SBS, short bowel syndrome.
Thus, forty subjects (sixteen males and twenty-four females), with a mean age of 14·8 (sd 6·8) years, participated in the present study. Underlying diagnoses were normally distributed and are shown in Table 1. Other diagnoses were long-segment Hirschsprung disease (n 1) and ischaemic small bowel of unknown origin (n 1). The mean residual bowel length was 70·8 cm, which corresponds with 26·5 % of the small bowel remaining. PN had been given for a median period of 110 (range 43–2345) d and all subjects were weaned off PN by the time of measurement. The clinical characteristics representing the first year of life are presented in Table 1.
Table 1 Clinical characteristics of the first year of life (n 40)
(Numbers, percentages, mean values and standard deviations, medians, minimum and maximum values)

SBS, short bowel syndrome; NEC, necrotising enterocolitis; CF, cystic fibrosis; GA, gestational age; SDS, standard deviation score, LOS, length of stay; min, minimum; max, maximum; SB, small bowel; ICV, ileocaecal valve; PN, parenteral nutrition; MEF, minimal enteral feeding; EN, enteral nutrition.
* A patient lost more bowels at the age of 11 years as result of strangulation and underwent a Bianchi procedure at the age of 12 years and was measured at 16 years.
Growth
Weight and height are presented in Table 2. The mean age of the children (twelve males and nineteen females) was 11·8 (sd 4·2) years. Their mean weight for age and height for age were significantly lower than reference values (P = 0·005 and P = 0·001, respectively). Mean weight for height and mean TH were normal. Mean height for age was significantly (P = 0·000) lower than TH. In total, 53 % were below their TH range.
Table 2 Weight and height
(Mean values and standard deviations, medians, minimum and maximum values)
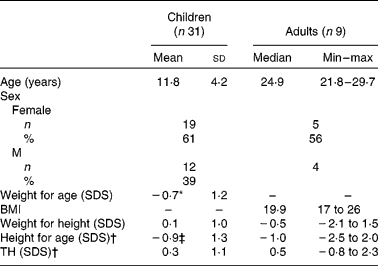
Min, minimum; max, maximum; SDS, standard deviations score; TH, target height.
* Mean values were significantly lower than reference value (P = 0·005).
† Mean values were significant different between height and TH in children (P = 0·000) and in adults (P = 0·008).
‡ Mean values were significantly lower than reference value (P = 0·001).
Owing to the small number of adults, the results are presented as median (range). Median age of the adults (four males and five females) was 24·9 (range 21·8–29·7) years. Median BMI and weight for height were normal. Median height for age was significantly lower than TH (Z = − 2·68, P = 0·008). In total, 78 % were below their TH range.
Body composition
For children, the mean of the sum of four skinfolds was − 0·9 (sd 1·0) SDS. Their mean BMC ( − 1·0 (sd 1·1) SDS) and mean LBM ( − 1·2 (sd 1·0) SDS) were significantly lower than reference values (P = 0·000). Only the mean BMDls ( − 0·47 (sd 1·2) SDS) was significantly lower than reference values (P = 0·036).
In adults, BMDls and BMDtb did not differ significantly from reference values. SDS of BMC, LBM and %BF of adults could not be calculated for lack of appropriate reference values. Table 3 reports the bone composition of all patients, as measured by DEXA.
Table 3 Bone composition measured by dual-energy X-ray absorptiometry
(Mean values and standard deviations, medians, minimum and maximum values)
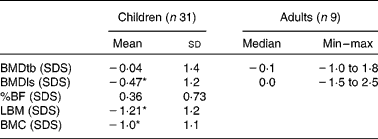
Min, minimum; max, maximum; BMDtb, bone mineral density of total body; SDS, standard deviation scores; BMDls, bone mineral density of lumbar spine; %BF, percentage body fat; LBM, lean body mass; BMC, bone mineral content.
* Mean values were significantly lower than the reference values (P < 0·05).
%BF calculated from DEXA and skinfold measurements are shown in Table 4. In three of the four adult males and five of the eleven male children had %BF below 10 %, indicating malnutrition. All the females, but one, had normal (15–25 %BF).
Table 4 Body composition
(Mean values and standard deviations, medians, minimum and maximum values)
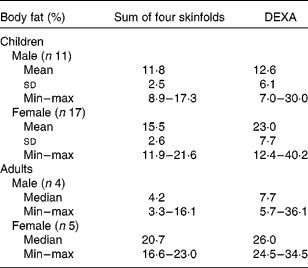
DEXA, dual-energy X-ray absorptiometry; min, minumum; max, maximum.
The limits of agreement of %BF for the two methods in children are shown in Fig. 2. Skinfolds underestimated %BF with 4·1 (95 % CI 1·97, 6·23). Paired-sample t tests showed significant differences in means between %BF measured by skinfolds and %BF measured by DEXA (P = 0·001).

Fig. 2 Bland–Altman plot percentage of body fat (%BF) skinfolds (sf) and dual-energy X-ray absorptiometry (DEXA).
Dietary intake
Dietary intakes are shown in Table 5. Mean energy intake was 8823 (sd 2433) kJ (2107 (sd 581) kcal), which is 91 (sd 28) % of the estimated average requirements. In all, seventeen subjects (45 %) had a energy intake more than 10 % below estimated average requirements and six (17 %) had a energy intake more than 10 % above estimated average requirements. A total of four subjects (10 %) were using enteral supplements (i.e. tube feeding). A total of nineteen (50 %) had a dietary calcium intake more than 10 % below recommended daily allowances.
Table 5 Dietary intake
(Mean values and standard deviations, medians, minimum and maximum values)

Min, minimum; max, maximum; EAR, estimated average requirements; RDA, recommended daily allowance; AI, adequate intake.
Defecation pattern
The results of the questionnaire are presented in Table 6. Stool frequency for all subjects (median 2 (range 0·3–7) per d) was significantly higher than that in the healthy population (median 1 (range 0·3–5); P = 0·000). A total of 35 % reported abnormal stool form (type 1, 6 and 7 of Bristol stool form scale) v. 2 % of the healthy population. Subjects self-estimated stool quantity was significantly higher (P = 0·014) than that for the normal population, and they also reported significantly more complaints such as bowel cramps, bloating and flatulence (P < 0·05).
Table 6 Defecation pattern
(Numbers, percentages, mean values and standard deviations)

Current health status and Tanner stages
Most children had Tanner stages corresponding with their age. One girl was in early puberty (age 9 years, Tanner stage 2) and two girls had delayed puberty (15 and 17 years old and both in Tanner stage 3). Most subjects had normal heart rates and blood pressure (data not shown). Standard neurological examination by the physician revealed no neurological problems.
Measurement results for the whole group did not differ from those for the subpopulation excluding subjects with cystic fibrosis (data not shown). Subjects with cystic fibrosis were in the same range as those with other underlying diagnoses.
Discussion
Increasing concern about morbidity following infantile bowel resection has resulted in intestinal rehabilitation programmes in different institutions(Reference Torres, Sudan and Vanderhoof38–Reference Sudan, Dibaise and Torres43). However, multidisciplinary data on long-term outcomes in patients with infantile SBS are still scarce. The present study was conducted to add to the knowledge on nutritional status and growth parameters after infantile SBS. More than half of the children and three-quarters of adults had not reached their TH range. Weight in general was normal for height and most subjects had normal %BF. LBM and BMC evaluated by DEXA were significantly below reference values in children.
Recently, we reported that the SDS for weight for age in the first year of life of these subjects were subnormal and had even declined significantly in the second- and third-quarterly terms(Reference Olieman, Tibboel and Penning9). From the results of the present study, it can be concluded that weight for age seems to revert to normal in the long run. SDS for height for age had also improved over the years, but were still significantly below reference values and TH. In contrast, Goulet et al. (Reference Goulet, Baglin-Gobet and Talbotec44) reported that the final height in fifty-seven children after 16 years follow-up generally was not different from their TH. As a possible explanation, Goulet et al. used Tanner's formula (1970) for the calculation of TH, which typically yields height values 4·5 cm shorter than those resulting from the calculation method that we used (Dutch growth study, 1997). Some other studies also found short stature (defined as < 50th percentile of height for age) in 60–90 % of children with SBS after weaning from PN(Reference Barksdale, Koehler and Yaworski45–Reference Gonzalez, Perez and Malpeli47). In contrast, several studies reported normal growth for most subjects(Reference Weber, Tracy and Connors48–Reference Leonberg, Chuang and Eicher50). The conflicting data seem to arise from differences in reference populations, definitions of short stature and moments of measurement.
The children in the present study showed reduced bone mineralisation only in the lumbar spine, which seems to suggest that only the trabecular bone, which is predominant in the lumbar spine, was affected(Reference Martin, Ng and Nicholson51). In contrast to children, the BMDls for the adults was normal. Leonberg et al. (Reference Leonberg, Chuang and Eicher50) also found subnormal BMC values, i.e. in four out of nine children with SBS. These values were established by single-photon absorptiometry in the forearm using other reference values(Reference Mazess and Cameron52) than that used by us. Dellert et al. (Reference Dellert, Farrell and Specker53) did not find subnormal BMC after adjusting the values for weight and height, but did when adjusting for age. It is not easy to compare results, as these researchers used another type of DEXA (Hologic) and studied two age-, sex- and race-matched controls per subject. Moreover, these controls were significantly heavier and taller than the children with SBS(Reference Dellert, Farrell and Specker53) – a finding most probably explained by either malabsorption or prolonged inadequate dietary intake in the subjects(Reference Ament54). The differences in BMC when control subjects and patients with SBS were matched for age are an indication for some sort of nutrient deficiency(Reference Ament54). This is also seen in patients with inflammatory bowel disease(Reference Boot, Bouquet and Krenning7). Vitamin D deficiency impairs Ca absorption and may explain low mineral content. Miyasaka et al. (Reference Miyasaka, Brown and Kadoura55) showed that some adolescents with SBS, who had poor growth, were vitamin D deficient and needed extra nutritional supplementation during puberty. Differences in BMC may reflect differences in either bone size or bone density(Reference Ahmed, Horrocks and Patterson56). Ahmed et al. (Reference Ahmed, Horrocks and Patterson56) suggested that children with inflammatory bowel disease often have small bones for age, as result of growth retardation. When they interpreted DEXA data adjusted for bone size, bone mass was generally found to be adequate(Reference Ahmed, Horrocks and Patterson56). It seems, therefore, that low BMC values in the present study can partially be explained by the short stature of the subjects with its inherent small bones.
Haderslev et al. (Reference Haderslev, Jeppesen and Sorensen57) found that PN-independent adults (mean age 50·6 years), who had undergone bowel resection a mean 11 years ago, had lower weight and mainly lower %BF compared to reference values. This holds true for only nine (22 %) of the subjects in the present study. Most of the children and female adults had normal weight for height and %BF. Two other studies also found normal weight for height and %BF(Reference Wu, Tang and Feng49, Reference Leonberg, Chuang and Eicher50).
The present study showed wide limits of agreement between outcomes of DEXA and skinfold thickness measurements, which indicates that these methods are not interchangeable. This finding is consistent with prior studies(Reference Haderslev and Staun58–Reference de Meer, Gulmans and Westerterp61). Skinfold thickness measurements are based on two assumptions. First, the thickness of subcutaneous adipose tissue reflects a constant proportion of total body fat; second, the sites selected for measurement represent the average thickness of subcutaneous adipose tissue(Reference Lukaski62). Moreover, body composition measured by skinfold thickness is based on a two-compartment model: fat-free mass and fat mass. DEXA is based on a three-compartment model: BMC, LBM and fat mass. The predictive equations used to calculate the body composition in the present study were developed and validated in healthy individuals, which might explain the wide levels of agreement. We have to realise that the degree to which subcutaneous adipose tissue reflects total body fat mass may change with age, sex, race and disease(Reference King, Wilson and Kotsimbos60, Reference Brodie, Moscrip and Hutcheon63, Reference Deurenberg64). It would seem, therefore, that DEXA is to be preferred.
The mean reported dietary intake of the subjects was lower than their average estimated intake. Dietary intake is difficult to measure and it is easily under or overrated(Reference Cade, Thompson and Burley65). Protein intake was high compared to the recommended daily allowances, but similar when compared to a Dutch food consumption survey(66). Moreover, 50 % of the patients had a dietary Ca intake 10 % below the recommended daily allowances. These results might be skewed, because oral supplementation of Ca supplements (i.e. calcium carbonate) was not taken into account. Assuming that the dietary records truly reflected dietary intake over the previous years, the lower dietary intake might explain the shorter stature and lower values of BMC of the subjects.
Several studies(Reference Heaton, Radvan and Cripps28, Reference Davies, Crowder and Reid67–Reference Heaton and O'Donnell70) showed that the Bristol stool scale form is correlated with whole-gut transit time and can be used to monitor change in intestinal function.
The subjects in the present study reported a higher frequency of stools than that reported by healthy aged-matched controls and 35 % had abnormal stools, which might indicate malabsorption and can partly explain lower BMC values. Moreover, they appeared to have complaints such as bowel cramps, bloating and flatulence significantly more often. The questionnaire was designed to ask what their normal bowel habits were, and we realise that this does not describe the bowel habits and changes over time. It does reveal, however, that SBS is associated with intestinal bowel dysfunction in the longer term.
The present study has limitations in its cross-sectional design, which causes age differences, in the absence of a functional test to determine the actual absorptive function of the bowel, in the absence of a hand X-ray to determine bone age and in the absence of measurement of vitamin D status. Moreover, subjects were identified from medical records and eligible patients could have been missed. Finally, the group is heterogeneous with respect to underlying diagnosis and remaining bowel lengths, which nevertheless is inherent to SBS.
On the other hand, the present study covered a long period, from 5 to 30 years after infantile SBS. This enabled us to describe the natural history of SBS into adulthood. As another strength, we used a unique combination of parameters to determine a broad spectrum of long-term effects in a relatively large group of patients.
Conclusion
Subjects in the present study had shorter stature, low BMC, but normal weight for height and %BF. This might be explained by the low energy intake and intestinal bowel dysfunction reported. These results show that continuing follow-up into adulthood is important even after subjects have reached nutritional autonomy. This way, low energy intake and intestinal bowel dysfunction might be identified early, enabling prevention of short stature by targeted nutritional management. Measurement of body composition is an essential aspect of providing optimal nutritional management and should preferably be done by DEXA.
Acknowledgements
The authors thank Jopie Sluimer (Erasmus Medical Center, Rotterdam, the Netherlands) for performing DEXA scans and Ko Hagoort (Erasmus Medical Center, Rotterdam, the Netherlands) for editing. J. F. O., J. C. E., H. I., T. L. v. d. H. and D. T. designed the study; J. F. O. and M. S. performed the experiments; J. F. O. and C. P. carried out data analysis; J. F. O. and C. P. prepared the manuscript; C. P., M. S., H. I., T. L. v. d. H., J. C. E., N. M. A. B. and D. T. carried out critical reviews of the manuscript. All the authors approved the final version of the manuscript. The authors state that the present study did not receive any funding. The authors declare no conflict of interest.












