Abstract
Down syndrome (DS) patients have an increased risk of developing pulmonary hypertension later in life compared to age-matched controls. The goal of this study was to determine if the incidence of persistent pulmonary hypertension of the newborn (PPHN) is also higher in neonatal DS patients compared to the general population. A retrospective chart review of DS patients admitted during a 3-year period to the neonatal intensive care unit was performed. DS patients with meconium aspiration syndrome, pulmonary infections, or pulmonary space-occupying lesions were excluded. DS patients were divided into four groups based on treatment and consisted of no intervention (A), supplemental oxygen (B,) mechanical ventilation use (C), and inhaled nitric oxide administration (D). Group D was defined as having PPHN. z test of the difference between sample and known population, chi-square, t-test, and analysis of variance with Tukey adjusted post hoc test were used for analysis. p < 0.05 was considered significant. A total of 58 patients met inclusion criteria. Twenty-four DS patients were in group A, 17 in group B, 10 in group C, and 7 in group D. There was no difference between the four groups for gender (males: 10, 5, 5, and 5, respectively), gestational age (36.4, 38.2, 36.4, and 36.4 weeks, respectively), weight (2.8, 3.0, 2.4, and 3.0 kg, respectively), or the presence of congenital heart defects (17, 10, 6, and 1, respectively). The estimated number of DS patients born in the state of Ohio during this period was 598; therefore, the incidence of PPHN in DS was 1.2%. The reported incidence of PPHN is 0.1%. The Reported incidence of PPHN was significantly lower versus the incidence of PPHN in DS (z = 2.7, p = 0.007). It was concluded that DS patients have an increased incidence of PPHN compared to historical controls regardless of baseline demographics.
Similar content being viewed by others
Children with Down syndrome (DS) have an increased risk for developing pulmonary hypertension. This may be due to multiple factors, including the presence of congenital heart disease with persistent left-to-right shunts [7, 24], chronic upper airway obstruction [15, 20], or abnormal pulmonary vasculature growth [8]. In addition, patients with DS and congenital heart disease seem to develop pulmonary hypertension at a faster rate and have persistent disease after cardiac surgery compared to non-DS patients with similar defects [6, 12]. Although damage to the pulmonary vasculature occurs over time, there also appears to be a subset of DS patients who develop pulmonary hypertension in the neonatal period [22].
Upper airway obstruction causing hypoventilation and heart defects with left-to-right shunts would not explain the development of pulmonary hypertension in neonates with DS. If there is an increased incidence of pulmonary hypertension in this neonatal population, then this would be consistent with the hypothesis that there are intrinsic factors in DS patients that place them at an increased risk for developing neonatal pulmonary hypertension.
The objective of this study was to document the incidence of persistent pulmonary hypertension of the newborn (PPHN) in the neonatal DS population and determine if this is higher than expected compared to the reported incidence.
Materials and Methods
The investigational review board at Columbus Children’s Hospital approved this study. Records of neonates born during a 3-year period from October 2002 to November 2005 with documented diagnosis of trisomy 21 and admitted to the neonatology intensive care unit (NICU) were reviewed. Demographics including gender, weight, gestational age, and the presence of cardiac defects were recorded. The echocardiogram was considered anatomically normal if the neonate had no intracardiac abnormalities or only had a patent ductus arteriosus and/or patent foramen ovale.
Neonates with meconium aspiration syndrome, pulmonary infections, or associated congenital malformations that may cause pulmonary hypertension such as pulmonary space-occupying lesions were excluded from this study; therefore, all cases of pulmonary hypertension reviewed were idiopathic in nature. PPHN was defined as being present if all three of the following criteria were met: (1) a right-to-left shunt at the ductal level or flattening of the interventricular septum in the absence of a patent ductus arteriosus by echocardiography, (2) use of greater than 50% supplemental oxygen, and (3) nitric oxide administration [25]. Other interventions used to treat hypoxemia were also recorded. Interventions included use of nasal cannula oxygen, continuous positive airway pressure, conventional ventilator, high-frequency ventilator, or extracorporeal membrane oxygenation (ECMO). The administration of nitric oxide, surfactant, or sildenafil was documented. Total ventilator days, total length of hospitalization, death during hospitalization, and use of home oxygen were also recorded. Patients were divided into four groups—A, B, C, and D. Group A patients had no interventions performed, group B patients were treated with nasal cannula or continuous positive airway pressure, group C patients were treated with mechanical ventilation, and group D patients were treated with inhaled nitric oxide (NO). Group D patients met criteria for PPHN.
Comparisons were performed between the four different groups. Finally, data from the State Department of Health and reported incidence data [10, 25] were used to determine if the incidence of PPHN was higher in neonates with DS compared to the general population.
Statistical Analysis
The effect of degree of groups on various measured data was tested with chi-square, t test, and analysis of variance (ANOVA) with Tukey adjusted post hoc paired comparisons performed when appropriate. z test of the difference between a sample proportion and a known population value was used to determine if the incidence of PPHN was different between DS and reported incidence data for the general population. A p value <0.05 was considered significant.
Results
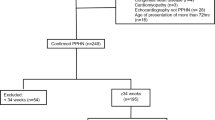
A total of 58 patients with the diagnosis of DS who met inclusion criteria were admitted to the NICU during the study period. Twenty-four patients (41%) were classified in group A, 17 (29%) in group B, 10 (17%) in group C, and 7 (12%) in group D. There were no significant differences in gender or the presence of complex congenital heart disease between the four groups of patients (Table 1). There was also no difference in gestational age in weeks (A, 36.4 ± 3.2; B, 38.2 ± 1.2; C, 36.4 ± 3.6; D, 36.4 ± 2.8; ANOVA F = 1.8, p = 0.16) or birth weight in kilograms (A, 2.8 ± 0.8; B, 3.0 ± 0.5; C, 2.4 ± 0.8; D, 3.0 ± 1.0; ANOVA F = 1.5, p = 0.23) between the four groups. Group A had 4 patients with prenatal risk factors (decrease fetal movements, maternal fever, and maternal GBBS+ ×2), group B had 7 patients (decrease fetal movements, maternal Klebsiella infection, maternal GBBS+, oligohydramnios ×2, maternal fever, and fetal heart rate decelerations), group C had 3 patients (fetal heart rate decelerations, oligohydramnios, and preeclampsia), and group D had 2 patients (oligohydramnios and bilateral pleural effusions). There was a nonsignificant association between risk factors and groups (chi-square = 3.04, p = 0.39). The 1- and 5-minute APGAR scores, respectively, were as follows: group A, 6.8 ± 1.6 and 8.0 ± 0.8; group B, 6.7 ± 1.8 and 8.4 ± 0.7; group C, 6.6 ± 1.3 and 8.0 ± 0.9; and group D, 3.7 ± 3.8 and 4.7 ± 3.1. Tukey adjusted test revealed significant differences between group D and all other groups for APGARS at both 1 minute (all, p < 0.02) and 5 minutes (all, p < 0.001).
In the seven patients with PPHN (group D), one patient had an atrioventricular septal defect, one had normal anatomy, and five had a patent ductus arteriosus. Oxygen saturation at hospital admission was 75.3 ± 19.0% for this group, which was significantly different compared to the other groups (ANOVA F = 8.0, p < 0.001). During the hospital course, three patients were treated with high-frequency ventilation (p = 0.001), one patient was placed on ECMO, two patients were treated with sildenafil (p = 0.002), and three patients treated with surfactant (p = 0.001). These treatments for the severe PPHN group were all significantly different compared to those of the other groups. ECMO use was not able to be statistically analyzed. The mean duration of mechanical ventilation was 11.6 ± 5.8 days, and the total hospitalization lasted a mean of 40.3 ± 30.9 days (Table 2). When the two patients who died (patients 3 and 7) were excluded, the length of hospitalization increased to 51.6 ± 21.6 days. The duration of mechanical ventilation was significantly longer in group D (11.6 ± 5.8 days) than in group C (5.3 ± 4.1 days) (ANOVA p = 0.001, Tukey p < 0.05). Length of hospital stay for the survivors was not significantly different between group D (51.6 ± 21.6 days) and group C (55.6 ± 33.7 days), but it was significantly longer for both group D and group C compared to that for groups B and A (B, 18.4 ± 16.6 days; A, 21.3 ± 17.2 days; ANOVA p < 0.001, Tukey p < 0.05). Two patients were discharged on supplemental oxygen and two patients died while hospitalized in group D.
During the 3-year study period, there were 79,757 live births in the seven-county central Ohio referral region and approximately 450,000 live births in the entire state of Ohio (Ohio State Department of Health). The national estimated number of DS births per live births is 13.29/10,000 [10]; therefore, the estimated number of DS cases for the seven-county region during this 3-year period is 106 DS patients, and that for the state is 598 DS patients. The incidence of PPHN in DS neonates for the seven-county region would thus be 6.60% (7/106) and for the state would be 1.17% (7/598). Since there are other hospitals in the state that would also treat DS patients with PPHN, the estimated state incidence of PPHN in DS is therefore a conservative estimate when using a single-center database. The reported incidence of PPHN in neonates is approximately 0.10% [25]. The estimated state incidence of 1.17% (z = 2.44, p = 0.007) and the estimated seven-county region incidence of 6.60% (z = 2.70, p = 0.003) of PPHN were both significantly higher compared to the reported incidence of 0.10% by z test of the difference between a sample population and a known population value.
Discussion
Pulmonary hypertension is a well-described phenomenon in DS patients [12, 16, 20, 22]. The cause of pulmonary hypertension in this population is multifactorial and may be due to both anatomical and physiological alterations in the pulmonary circulation. Decreased alveolar density has been reported in some DS patients compared to non-DS controls, and this may be an etiology of the pulmonary hypertension [8]; however, the data are conflicting [26]. Chronic obstructive upper airway obstruction would cause hypoxemia that could eventually lead to pulmonary hypertension in older DS patients [14, 20]; however, this would not be a cause of PPHN. DS patients with congenital heart disease such as an atrioventricular septal defect are also exposed to persistent left-to-right blood flow shunts [9, 12]. Over time, this exposure to increased pulmonary blood flow would result in increased shear stress on endothelial cells and might impair production of NO, a potent vasodilator. All of the previously discussed etiologies would require a period of time to result in clinically detectable pulmonary hypertension and thus are unlikely to be involved in the pathogenesis of PPHN in neonatal DS patients. This study documented a significantly increased incidence of PPHN in neonatal DS patients compared to the reported incidence. Furthermore, there were no differences in demographics or risk factors between the DS groups in this study that would explain the presence of PPHN.
The increased incidence of PPHN in DS patients suggests that there may be something intrinsically related to DS that put these patients at increased risk for neonatal pulmonary hypertension. When comparing patients with DS and congenital heart disease with non-DS patients with similar defects, the DS patients appear to develop pulmonary hypertension at a faster rate and have persistence of pulmonary hypertension after cardiac surgery [12]. A recent study showed that DS patients may have an abnormal production of NO but respond appropriately to exogenous NO administration in the peripheral circulation [5]. Another study documented less pulmonary vasodilation response to NO in DS patients versus controls in the cardiac catheterization laboratory [4].
Thus, a genetic factor in this population may contribute to and explain the increased incidence of PPHN in this population. Recently described genetic polymorphisms, such as eNOS [13, 23, 27], ACE [1–3], AT1R [11, 18], and BMPR2 [17, 19, 21], have been associated with abnormal NO production and increased risk for systemic and pulmonary hypertension in the general population. One study testing for BMPR2 mutation in patients with congenital heart disease and pulmonary hypertension showed an occurrence in a subset of DS patients [19]. Therefore, the presence of an increased incidence of PPHN in DS patients is consistent with the hypothesis that there is an intrinsic cause for the development of PPHN in DS patients.
There are multiple limitations of this study. It is a retrospective study with all the inherent shortcomings associated with such a design. There were also assumptions made about the number of DS patients born during this period, but the incidence data were obtained from reliable state sources and national registries [10]. The data analyzed used only well-described cases of PPHN and a statewide incidence (1.17%) compared to the reported incidence in the general population to be as conservative as possible. Even with these very conservative assumptions and definitions, there was a significant difference in the incidence of PPHN in the DS patients compared to the reported incidence in the general population. This data included only DS patients admitted to one center, and there may be some DS patients born in the seven-county referral area who may have been treated at other institutions for PPHN; however, this error would only tend to underestimate the incidence of PPHN in DS patients. The true incidence of PPHN in DS patients most likely lies somewhere between the statewide estimate of 1.17% and the seven-county estimate of 6.60%.
In conclusion, neonatal DS patients have a greater incidence of PPHN compared to the general population regardless of baseline demographics or risk factors. This finding is consistent with an intrinsic predisposition for PPHN among DS patients, but further studies are required to determine these possible intrinsic predispositions.
References
Abraham WT, Raynolds MV, Badesch DB, et al. (2003) Angiotensin-converting enzyme DD genotype in patients with primary pulmonary hypertension: increased frequency and association with preserved haemodynamics. J Renin Angiotensin Aldosterone Syst 4:27–30
Abraham WT, Raynolds MV, Gottschall B, et al. (1995) Importance of angiotensin-converting enzyme in pulmonary hypertension. Cardiology 86(Suppl 1):9–15
Ahsan A, Ram R, Baig MA, Pasha MA (2004) ACE I allele and eNOS G allele crosstalk may have a role in chronic obstructive pulmonary disease. Clin Biochem 37:1037–1040
Cannon BC, Feltes TF, Fraley JK, et al. (2005) Nitric oxide in the evaluation of congenital heart disease with pulmonary hypertension: factors related to nitric oxide response. Pediatr Cardiol 26:565–569
Cappelli-Bigazzi M, Santoro G, Battaglia C, et al. (2004) Endothelial cell function in patients with Down’s syndrome. Am J Cardiol 94:392–395
Chi TPLKJ (1975) The pulmonary vascular bed in children with Down syndrome. J Pediatr 86:533–538
Clapp S, Perry BL, Farooki ZQ, et al. (1990) Down’s syndrome, complete atrioventricular canal, and pulmonary vascular obstructive disease. J Thorac Cardiovasc Surg 100:115–121
Cooney TP, Thurlbeck WM (1982) Pulmonary hypoplasia in Down’s syndrome. N Engl J Med 307:1170–1173
Hasegawa N, Oshima M, Kawakami H, Hirano H (1990) Changes in pulmonary tissue of patients with congenital heart disease and Down syndrome: a morphological and histochemical study. Acta Paediatr Jpn 32:60–66
Improved national prevalence estimates for 18 selected major birth defects—United States, 1999–2001 (2006) Morbid Mortal Weekly Rep 54:1301–1305
Jugdutt BI, Menon V (2004) AT1 receptor blockade limits myocardial injury and upregulates AT2 receptors during reperfused myocardial infarction. Mol Cell Biochem 260:111–118
Kawai T, Wada Y, Enmoto T, et al. (1995) Comparison of hemodynamic data before and after corrective surgery for Down’s syndrome and ventricular septal defect. Heart Vessels 10:154–157
Kunnas TA, Lehtimaki T, Laaksonen R, et al. (2002) Endothelial nitric oxide synthase genotype modulates the improvement of coronary blood flow by pravastatin: a placebo-controlled PET study. J Mol Med 80:802–807
Lefaivre JF, Cohen SR, Burstein FD, et al. (1997) Down syndrome: identification and surgical management of obstructive sleep apnea. Plast Reconstr Surg 99:629–637
Levine OR, Simpser M (1982) Alveolar hypoventilation and cor pulmonale associated with chronic airway obstruction in infants with Down syndrome. Clin Pediatr (Philadelphia) 21:25–29
Lindberg L, Olsson AK, Jogi P, Jonmarker C (2002) How common is severe pulmonary hypertension after pediatric cardiac surgery? J Thorac Cardiovasc Surg 123:1155–1163
Machado RD, Pauciulo MW, Thomson JR, et al. (2001) BMPR2 haploinsufficiency as the inherited molecular mechanism for primary pulmonary hypertension. Am J Hum Genet 68:92–102
Mettimano M, Romano-Spica V, Ianni A, et al. (2002) AGT and AT1R gene polymorphism in hypertensive heart disease. Int J Clin Pract 56:574–577
Roberts KE, McElroy JJ, Wong WP, et al. (2004) BMPR2 mutations in pulmonary arterial hypertension with congenital heart disease. Eur Respir J 24:371–374
Rowland TW, Nordstrom LG, Bean MS, Burkhardt H (1981) Chronic upper airway obstruction and pulmonary hypertension in Down’s syndrome. Am J Dis Child 135:1050–1052
Runo JR, Vnencak-Jones CL, Prince M, et al. (2003) Pulmonary veno-occlusive disease caused by an inherited mutation in bone morphogenetic protein receptor II. Am J Respir Crit Care Med 167:889–894
Shah PS, Hellmann J, Adatia I (2004) Clinical characteristics and follow up of Down syndrome infants without congenital heart disease who presented with persistent pulmonary hypertension of newborn. J Perinat Med 32:168–170
Shimasaki Y, Yasue H, Yoshimura M, et al. (1998) Association of the missense Glu298Asp variant of the endothelial nitric oxide synthase gene with myocardial infarction. J Am Coll Cardiol 31:1506–1510
Suzuki K, Yamaki S, Mimori S, et al. (2000) Pulmonary vascular disease in Down’s syndrome with complete atrioventricular septal defect. Am J Cardiol 86:434–437
Walsh-Sukys MC, Tyson JE, Wright LL, et al. (2000) Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics 105:14–20
Wilson SK, Hutchins GM, Neill CA (1979) Hypertensive pulmonary vascular disease in Down syndrome. J Pediatr 95: 722–726
Wolff B, Grabe HJ, Schluter C, et al. (2005) Endothelial nitric oxide synthase Glu298Asp gene polymorphism, blood pressure and hypertension in a general population sample. J Hypertens 23:1361–1366
Author information
Authors and Affiliations
Corresponding author
Additional information
Abstract presented to the American Academy of Pediatrics, October 2006
Rights and permissions
About this article
Cite this article
Cua, C.L., Blankenship, A., North, A.L. et al. Increased Incidence of Idiopathic Persistent Pulmonary Hypertension in Down Syndrome Neonates. Pediatr Cardiol 28, 250–254 (2007). https://doi.org/10.1007/s00246-006-0011-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-006-0011-6