Abstract
Haemodynamic factors play an important role in the etiology of cerebral lesions in preterm infants. Respiratory distress syndrome (RDS), a common problem in preterms, is strongly related with low and fluctuating arterial blood pressure. This study investigated the relation between mean arterial blood pressure (MABP), fractional cerebral oxygen saturation (ScO2) and fractional (cerebral) tissue oxygen extraction (FTOE), a measure of oxygen utilisation of the brain, during the first 72 h of life. Thirty-eight infants (gestational age < 32 week) were included, 18 with and 20 without RDS. Arterial oxygen saturation (SaO2), MABP and near infrared spectroscopy-determined ScO2 were continuously measured. FTOE was calculated as a ratio: (SaO2–ScO2)/SaO2. Gestational age and birth weight did not differ between groups, but assisted ventilation and use of inotropic drugs were more common in RDS infants (P<0.01). MABP was lower in RDS patients (P<0.05 from 12 up to 36 h after birth), but increased in both groups over time. ScO2 and FTOE were not different between groups over time, but in RDS infants ScO2 and FTOE had substantial larger variance (P<0.05 at all time points except at 36–48 h for ScO2 and P<0.05 at 12–18, 18–24, 36–48 and 48–60 h for FTOE). During the first 72 h of life, RDS infants showed more periods of positive correlation between MABP and ScO2 (P<0.05 at 18–24, 24–36 36–48 48–60 h) and negative correlation between MABP and FTOE (P<0.05 at 18–24, 36–48 h). Although we found that the patterns of cerebral oxygenation and extraction in RDS infants were not different as compared to infants without RDS, we suggest that the frequent periods with possible lack of cerebral autoregulation in RDS infants may make these infants more vulnerable to cerebral damage.
Similar content being viewed by others
Introduction
The etiology of cerebral lesions such as periventricular/intraventricular haemorrhages (PIVH) and/or ischemic lesions such as periventricular leukomalacia are not fully understood. Although probably multifactorial, haemodynamic factors seem to play an important role here (Volpe 2001; Dammann and Leviton 1997; Osborn et al. 2003; Hunt et al. 2004). Previous studies reported a strong association between preterm infants with the lowest arterial blood pressure and the occurrence of PIVH. These findings suggest that underperfusion and subsequent deoxygenation with or without disturbances in the autoregulatory ability of the cerebral circulation is an important etiological factor for cerebral damage in these sick preterm infants (Miall-Allen et al. 1987; Bada et al. 1990).
Severe respiratory distress syndrome (RDS), a common and serious problem in the extremely preterm baby, is strongly related with low blood pressure and fluctuations in perfusion of the immature brain. Moreover, RDS is a well recognised risk factor for PIVH (Perlman et al. 1983; van Bel et al. 1987; Volpe 2001; Krediet et al. 2002). We therefore hypothesize that preterm infants with severe RDS have lower and less stable arterial blood pressures as compared to preterm infants without RDS, leading to disturbances of oxygenation of the brain in the first days of life.
To investigate this hypothesis we simultaneously monitored both arterial blood pressure and near infrared spectroscopy measured cerebral oxygenation and oxygen extraction in 38 preterm infants admitted consecutively with or without severe RDS during the first 72 h of life (Tsuji et al. 2000; Hintz 2001; Naulaers et al. 2002; Naulaers 2003).
Methods
Patient population
Thirty eight infants, 18 with RDS and 20 without RDS (No-RDS), with a gestational age (GA) of less than 32 completed weeks, consecutively admitted to the neonatal intensive care unit (NICU) of the Wilhelmina Children’s Hospital, were included in the present study. Infants with PIVH at birth, congenital malformations or infants older than 12 h after birth were excluded. Informed parental consent was obtained in all cases. The Medical Ethical Committee of the University Medical Centre Utrecht approved the present study.
Clinical data
Obstetrical and intrapartum data were collected from the hospital records. Neonatal data were collected prospectively. The arterial oxygen saturation (SaO2) was continuously measured by pulse oxymetry on a limb. Arterial blood pressure was monitored continuously by an indwelling arterial catheter (umbilical, tibial or radial artery) in all infants. Blood pressure support was started according to the decision of the attending neonatologist as indicated by the guidelines used in our NICU (e.g. starting blood pressure support if the mean arterial blood pressure in mmHg was lower than the gestational age in weeks). A blood pressure support scoring system, depending on the intensity of the treatment, was used to assess the intensity of blood pressure support (Krediet et al. 2002): Score 0: no support; score 1: volume expansion and/or dopamine ≤ 5 μg/kg/min; score 2: dopamine >5≤10 μg/kg/min; score 3: dopamine > 10 μg/kg/min or dopamine and dobutamine ≤ 10 μg/kg/min; score 4: dopamine + dobutamine > 10 μg/kg/min; score 5: additional adrenaline and/or corticosteroids.
RDS was defined as respiratory distress in a preterm infant with signs of RDS on the X-ray, the need of assisted ventilation and surfactant therapy (at least one gift). Treatment decisions were made by the attending neonatologist: ventilator settings were adjusted according to clinical and laboratory parameters (arterial pCO2 40–50 mmHg; arterial pO2 60–80 mmHg) and surfactant was administered according to the guidelines used in our NICU.
Monitoring cerebral tissue oxygenation
Because movement artefacts of the sensor fixed on the head of the baby prohibits reliable monitoring of changes in oxygenated, deoxygenated and total haemoglobin (HbO2, HbD, HbTot) using near infrared spectroscopy (NIRS) in the clinical situation during longer periods of time, we used the fractional cerebral oxygenation as a reliable estimator for changes in tissue cerebral oxygenation (Toet et al. 2005). Because absolute values are provided here, ScO2 is less dependent on movement artefacts and comparisons over time are possible (Naulaers et al. 2002; Naulaers 2003). Fractional cerebral tissue oxygen extraction (FTOE) can then be calculated from ScO2 and arterial oxygen saturation (SaO2) (see also below).
We used the near infrared spectrometer (INVOS 4100, Somanetics Corp., Troy, Mich). A transducer containing a light emitting diode and two distant sensors was attached to the fronto-parietal side of the neonatal skull. ScO2 was calculated from the differential signal obtained from these two sensors, expressed as the venous-weighted percent oxygenated haemoglobin [HbO2/HbTot (HbTot=HbO2 + HbD)] (Wyatt et al. 1986; Edwards et al. 1988; Pryds et al. 1990; Naulaers et al. 2002; Naulaers 2003).
To investigate the balance between oxygen delivery and oxygen consumption, a relative fractional tissue oxygen extraction measurement can be formulated as a ratio: (SaO2−ScO2)/SaO2. An increase in FTOE reflects an increase of the oxygen extraction by brain tissue suggesting a higher consumption than delivery of oxygen. On the other hand, a decrease of FTOE suggest less utilisation of oxygen by brain tissue in comparison with the supply (Naulaers et al. 2002; Naulaers 2003).
Study design
Monitoring and recording of the arterial blood pressure, heart rate, SaO2 and ScO2 were started as soon as possible after birth up to 72 h of life. All variables were collected simultaneously and stored on a personal computer for offline analysis (software: Poly 5, Inspektor Research Systems, Amsterdam NL), with a sampling frequency of 10 Hz.
The arterial haemoglobin concentration was measured daily, or more frequent if indicated. Arterial blood gasses were measured every 4 h in both groups during the first 24 h or more frequent if necessary and less frequent between 24 and 72 h of life if the clinical situation was stable.
Cranial ultrasound studies were performed shortly after birth or at admission and repeated every 24 h during the first 3 days (or more frequently if indicated), at the end of the first week and weekly thereafter by the attending neonatologist. PIVH was graded, as reported previously (de Vries et al. 1998): Grade I: small germinal layer haemorrhage; Grade IIa: germinal layer haemorrhage plus IVH, filling the lateral ventricle < 50%; Grade IIb: large IVH filling the lateral ventricle > 50%; Grade III: IVH associated with (unilateral) parenchymal involvement owing to venous of haemorrhagic infarction. The presence or absence of thermodynamically significant patent ductus arteriosus (PDA) was investigated at least daily or more frequently if appropriate, according to standard echocardiographic indices.
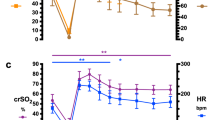
To reduce the number of data to a more acceptable amount and to be able to make comparisons between the various postnatal time points, a period of 60 min of reliable monitoring was taken from each patient between the following time windows: 6–12, 12–18, 18–24, 24–36, 36–48, 48–60 and 60–72 h after birth. Preferably, we selected 60 min halfway the above-mentioned time window, without medical or nursing interventions. In a minority of cases these interventions forced us to choose a stable period with a slightly different time frame. The mean values of the mean arterial blood pressure (MABP) (mmHg), SaO2 (%), ScO2 (%), FTOE (1/1), arterial haemoglobin concentration (mmol/l) and arterial pCO2 (mmHg) were calculated over each period of 60 min.
Statistical analysis
Data are summarised as mean values ± SD or as median values and ranges where appropriate. Student’s t test or chi-square test compared patient characteristics and differences in blood pressure support score. The Mann-Whitney U test and the Levene’s test for equality of variances were used to assess differences between the groups at the various time points; differences between MABP, ScO2 and FTOE within groups (shown as Box-and-Whisker Plots) were evaluated by ANOVA for repeated measurements to assess changes within groups over time. Adjustments for multiple comparisons were made by a Bonferroni post hoc test. To obtain an indication for a possible relationship between MABP on the one hand and ScO2 or FTOE on the other hand, correlation coefficients using simple linear regression analysis were performed over the four 15 min periods during each 60 min period (see also study design). A significant correlation coefficient was supposed to be a clinically relevant finding indicating a passive relationship between MABP on the one hand and ScO2 or FTOE (r>0.50) and r<−0.50, respectively) on the other. A P<0.05 was considered statistically significant. For statistical analysis SPSS version 12.0 was used.
Results
Clinical characteristics
The clinical characteristics of the RDS and No-RDS groups are shown in Table 1. Gestational age and birth weight were not significantly different between groups. Assisted ventilation was significantly more often needed in RDS infants (100%) as compared to No-RDS infants (P<0.05). Eight RDS patients (44.4%) needed therapy (indomethacin) for ductus arteriosus closure in the first 72 h compared to none of the No-RDS patients. The occurrence of PIVH was higher in the RDS group [n=7 (39%) in RDS-infants; n=3 (15%) in No-RDS-infants]. Severe PIVHs were exclusively found in the RDS group [five vs. none Grade IIa PIVH in No-RDS infants (P<0.01)]. Blood pressure support was significantly more needed in the RDS group as compared to the No-RDS group (P<0.01).
Patterns of MABP, ScO2 and FTOE
During the first 72 h of life the MABP increased significantly in both groups: from 33 mmHg in the 6–12 h period to 38 mmHg in the 60–72 h period in the RDS group and from 35 mmHg to 41 mmHg in the No-RDS group [significant at 6–12 vs. 60–72 h period (P<0.05)]. The MABP was lower in RDS infants as compared to No-RDS infants [significant at 12–18 and 18–24 h (P<0.05)]. The ScO2, as a measure of cerebral oxygenation, was stable in both groups over time. The variance of the ScO2 in the RDS group was however considerably larger [significant at all time periods except 24–36 h (P<0.05)]. The FTOE, indicating cerebral O2 extraction, showed a small rise in RDS infants over time (from 0.25 to 0.28), and a small decrease in No-RDS infants (from 0.28 to 0.26); however, no significance was reached. FTOE in RDS infants showed a larger variance as compared to No-RDS infants [significance was reached at 12–18, 18–24, 36–48 and 48–60 h (P<0.05)]. MABP, ScO2 and FTOE (medians and ranges) are shown in Fig. 1.
Figures 2a, b and 3a, b show the individual correlation coefficients between MABP and ScO2 and between MABP and FTOE in the various time periods. A correlation coefficient was calculated every 15 min during the 60 min period in each infant. As shown in Fig. 2a and b, there were more 15-min periods with a positive correlation between MABP and ScO2, as well as a negative correlation between MABP and FTOE in the RDS group in comparison with the No-RDS group. Figures 2c and 3c show the percentage of a significant correlation coefficient (r>0.50) between MABP and ScO2 and between MABP and FTOE (r<−0.50) during the various time periods in both groups. There were more periods of a significant correlation at all points of time in RDS infants. Significance of these parameters in RDS versus No-RDS infants was reached for MABP and ScO2 at 18–24, 24–36, 36–48 and 48–60 h and for MABP and FTOE at 18–24 and 36–48 h.
Individual correlation coefficients between mean arterial blood pressure (MABP) and fractional cerebral oxygenation (ScO 2 ) in No-RDS patients (n=20) (a) and RDS patients (n=18) (b). Four individual Pierson correlation coefficients were calculated (every 15 min) during each 60 min periods (6–12, 12–18, 18–24, 24–36, 36–48, 48–60, 60–72 h of life) A correlation coefficient r>0.50 was significant and supposed to be relevant. c Percentage of a significant correlation coefficient (r>0.50) in RDS and No-RDS infants
Individual correlation coefficients between mean arterial blood pressure (MABP) and fractional tissue oxygen extraction (FTOE) in No-RDS patients (n=20) (a) and RDS patients (n=18) (b). Four individual correlation coefficients were calculated (every 15 min) during each 60 min periods (6–12, 12–18, 18–24, 24–36, 36–48, 48–60, 60–72 h after birth) A correlation coefficient r<−0.50 was significant and supposed to be relevant. c Percentage of a significant correlation coefficient (r<−0.50) in RDS and No-RDS infants
Two representative individual examples of the relations between MABP and ScO2, and MABP and FTOE respectively as a function of time (60 min), which were derived between 18–24 h of one RDS infant and of one No-RDS infant are shown in Fig. 4a and b. From these individual tracings it is clear that in the RDS-infant (Fig. 4a) ScO2 and FTOE follow the fluctuations in MABP, strongly suggesting a correlation between MABP on the one hand and ScO2 and FTOE on the other (see also correlation plots in Fig. 4a). The tracing of the No-RDS-infant, on the other hand, shows an apparent independency of the ScO2 and FTOE patterns from the pattern of MABP (Fig. 4b). The individual differences shown in Fig. 4 are illustrative for the differences in the MABP versus ScO2/FTOE relationships between both groups.
Representative simultaneous tracings of mean arterial blood pressure (MABP), fractional cerebral oxygenation (ScO 2 ) and fractional tissue oxygenation extraction (FTOE) during a 60 min period between 18–24 h of a RDS infant (a) and a No-RDS infant (b). The related scatter plots of the MABP–ScO2 and the MABP–FTOE relationships are depicted on the upper part of the graph for both infants
Patterns of haemoglobin and pCO2
The mean haemoglobin concentrations were not significantly different in time or between groups. All values at any time in both groups were always in the normal range (8.5–10.7 mmol/l). Although the pCO2 was always within the normal range (40–50 mmHg) in all patients, the mean pCO2 in RDS patients was significantly higher at the time periods 36–48, 48–60 and 60–72 h.
Discussion
The results of the present paper show lower arterial blood pressures in infants with severe RDS during the first 36 h of life. Moreover, in contrast with the infants without RDS, a substantial need for blood pressure support was often needed in RDS infants to prevent frank hypotension in this group. This confirms earlier studies of our group and others, revealing lower blood pressures and, in particular, a significantly increased need for blood pressure support in infants with RDS. Moreover, these studies also showed that severity of RDS was linked with blood pressure and the need for blood pressure support (Krediet et al. 2002; van Bel et al. 2004).
There is no direct relation between NIRS-measured cerebral tissue oxygenation and/or cerebral oxygen extraction on the one hand and arterial blood pressure on the other hand, when relying on averaged values obtained over a longer period of time (60 min) and derived from relatively stable tracings. These findings are in line with a recent study of Kissack et al. (2004). However, our results also revealed substantial and often significantly larger variances of ScO2 and FTOE in RDS infants as compared to infants without RDS, suggesting more fluctuations of ScO2 and FTOE in RDS infants (see also Fig. 1).
Even more important was that a more detailed analysis of the actual tracings (see also Fig. 2, 3, 4) showed that infants suffering from RDS had significantly more periods of a positive and a negative relationship between MABP and ScO2, and MABP and FTOE respectively, during all periods of time studied, as compared to infants without RDS. Tsuji et al. (2000) already reported that critically ill preterm infants often exhibited a correlation between cerebral oxygenation and MABP, but they did not investigate this correlation in relation with RDS. These investigators and others (Ozdemir et al. 1997; Tsuji et al. 2000) suggest that this indicates an impairment of the cerebral-vascular autoregulatory ability causing substantial fluctuations in cerebral perfusion and oxygenation. The examples in Fig. 4, which are representative for the patterns of MABP, ScO2 and FTOE in RDS or No-RDS infants respectively are indeed very suspect for an impairment in cerebral autoregulation in RDS infants, but do not prove it, since the present study measured cerebral oxygenation and extraction and not actual brain blood flow.
Nonetheless, we feel that NIRS-determined ScO2 and FTOE may help to elucidate whether or not RDS is causally related to brain injury and in particular with the occurrence of (severe) PIVH (Tsuji et al. 2000; Dasgupta and Gill 2003). Having made the above mentioned assumptions regarding the NIRS measured oxygenation (ScO2) and oxygen extraction (FTOE), it must be admitted that NIRS is a non invasive method that reliably reflects changes in intracranial mixed saturation, but that no reliable absolute values are provided (Ozdemir et al. 1997; Dasgupta and Gill 2003; Kissack et al. 2004). The same is true for the NIRS-measured FTOE, which can be merely used to assess changes in oxygen extraction of the brain, although Naulaers (2003) found a close correlation between FTOE and actual fractional oxygen extraction in a piglet model. Despite the fact that absolute values cannot be provided by this method, signs of cerebral hypoxia as indicated by a decrease in ScO2 and increase in FTOE provide important information in the unstable preterm infant.
It may be possible that the lower arterial blood pressures in infants with severe RDS and the frequently occurring positive correlation between MABP and cerebral oxygenation and/or cerebral oxygen extraction in these infants are merely associations, not directly linked to RDS, being solely an expression of the severity of illness. We speculate that there are reasons to assume that these findings may be causative and related to the occurrence of RDS. In earlier studies of our group, in which we reported lower arterial blood pressures and increased blood pressure support (Krediet et al. 2002; van Bel et al. 2004), we found a close relationship between blood pressure and plasma-cyclic guanosine monophosphate (cGMP)-levels with significantly higher plasma cGMP concentrations in RDS-infants. Moreover, severity of RDS was positively linked with plasma cGMP-levels in these studies. Since cGMP mediates endothelium-dependent relaxation of the vascular bed, this may explain the diminished vascular resistance often seen in sick preterm RDS infants (Kluckow and Evans 1996) with subsequent lower blood pressures and increased need for blood pressure support (Kluckow and Evans 1996). We also found evidence that the involvement of pro-inflammatory processes in RDS (Ozdemir et al. 1997) with an increased expression of inducible haeme oxygenase and consequent production of carbon monoxide is responsible for the increased plasma levels of vasodilatory cGMP (van Bel et al. 2002; van Bel et al. 2004).
We have to take into account that other factors often associated or related with RDS which may influence haemodynamics, and by that cerebral oxygenation, were not investigated. Among these the most important factors are probably PDA, the incidence of which was significantly higher in the RDS-infants, and mechanical ventilation-induced haemodynamic alterations such as changes in left ventricular output (Kissack et al. 2004). With respect to PDA, earlier studies showed that this was not always a central factor in relation to blood pressure (Krediet et al. 2002; van Bel et al. 2004; Kissack et al. 2004). Moreover, in our clinical practice we are very active and aggressive with respect to early diagnosis and treatment of PDA in infants suffering from RDS. We did not measure (left) ventricular output and the effects of ventilation, PDA and indomethacin use were not studied in detail. We are therefore not informed about the importance of these variables in our observations. In particular, the effects of indomethacin may be rather prolonged and may therefore have influenced our measured variables. To fully appreciate these effects a more detailed study will be necessary. Finally, no clinical relevant differences in arterial carbon dioxide levels were detected between infants with and without RDS. The levels were measured every 4 h or more frequent if necessary in the first 24 h of life and at least twice a day thereafter and were always within the physiological range and within narrow limits. However, even small changes in arterial pCO2 may have an effect on cerebral haemodynamics and by that on cerebral oxygenation and/or oxygen extraction.
In summary, this study showed lower blood pressures in infants with RDS, and a substantial need for blood pressure support in these infants as compared to infants without RDS. Moreover, there was a significantly larger range of cerebral oxygenation and cerebral oxygen extraction and a stronger relation between these cerebral parameters and arterial blood pressure in infants suffering from RDS in comparison with No-RDS infants. This suggests more frequent periods of lack of cerebral-vascular autoregulation in these RDS infants as compared to No-RDS infants. This may explain the predisposition to brain damage, in particular PIVH, in infants with RDS. These findings further underscore the importance of monitoring oxygenation and extraction in the critically ill preterm infant with RDS.
Abbreviations
- FTOE:
-
Fractional (cerebral) tissue oxygen extraction
- MABP:
-
Mean arterial blood pressure
- NIRS:
-
Near infrared spectroscopy
- RDS:
-
Respiratory distress syndrome
- PDA:
-
Persistent ductus arteriosus
- PIVH:
-
Periventricular/intraventricular haemorrhage
- SaO2 :
-
Arterial oxygen saturation
- ScO2 :
-
Fractional cerebral oxygen saturation
Reference
Bada HS, Korones SB, Perry EH, Arheart KL, Ray JD, Pourcyrous M, Magill HL, Runyan W III, Somes GW, Clark FC (1990) Mean arterial blood pressure changes in premature infants and those at risk for intraventricular hemorrhage. J Pediatr 117:607–614
van Bel F, Van de Bor M, Stijnen T, Baan J, Ruys JH (1987) Aetiological role of cerebral blood-flow alterations in development and extension of peri-intraventricular haemorrhage. Dev Med Child Neurol 29:601–614
van Bel F, Valk L, Uiterwaal CS, Egberts J, Krediet TG (2002) Plasma guanosine 3′,5′-cyclic monophosphate and severity of peri/intraventricular haemorrhage in the preterm newborn. Acta Paediatr 91:434–439
van Bel F, Latour V, Vreman HJ, Wong RJ, Stevenson DK, Steendijk P, Egberts J, Krediet TG (2004) Is carbon monoxide-mediated cyclic guanosine monophosphate production responsible for low blood pressure in neonatal respiratory distress syndrome? J Appl Physiol 98:1044–1049
Dammann O, Leviton A (1997) Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res 42:1–8
Dasgupta SJ, Gill AB (2003) Hypotension in the very low birthweight infant: the old, the new, and the uncertain. Arch Dis Child Fetal Neonatal Ed 88:F450–F454
Edwards AD, Wyatt JS, Richardson C, Delpy DT, Cope M, Reynolds EO (1988) Cotside measurement of cerebral blood flow in ill newborn infants by near infrared spectroscopy. Lancet 2:770–771
Hintz SR (2001) Near-infrared spectroscopy: neonatal and perinatal applications. Neo Rev 2:e22–e28
Hunt RW, Evans N, Rieger I, Kluckow M (2004) Low superior vena cava flow and neurodevelopment at 3 years in very preterm infants. J Pediatr 145:588–592
Kissack CM, Garr R, Wardle SP, Weindling AM (2004) Cerebral fractional oxygen extraction in very low birth weight infants is high when there is low left ventricular output and hypocarbia but is unaffected by hypotension. Pediatr Res 55:400–405
Kluckow M, Evans N (1996) Relationship between blood pressure and cardiac output in preterm infants requiring mechanical ventilation. J Pediatr 129:506–512
Krediet TG, Valk L, Hempenius I, Egberts J, Van Bel F (2002) Nitric oxide production and plasma cyclic guanosine monophosphate in premature infants with respiratory distress syndrome. Biol Neonate 82:150–154
Miall-Allen VM, de Vries LS, Whitelaw AG (1987) Mean arterial blood pressure and neonatal cerebral lesions. Arch Dis Child 62:1068–1069
Naulaers G (2003) Non-invasive measurement of the neonatal cerebral and splanchnic circulation by near-infrared spectroscopy. University Press, Leuven
Naulaers G, Morren G, Van Huffel S, Casaer P, Devlieger H (2002) Cerebral tissue oxygenation index in very premature infants. Arch Dis Child Fetal Neonatal Ed 87:F189–F192
Osborn DA, Evans N, Kluckow M (2003) Hemodynamic and antecedent risk factors of early and late periventricular/intraventricular hemorrhage in premature infants. Pediatrics 112:33–39
Ozdemir A, Brown MA, Morgan WJ (1997) Markers and mediators of inflammation in neonatal lung disease. Pediatr Pulmonol 23:292–306
Perlman JM, McMenamin JB, Volpe JJ (1983) Fluctuating cerebral blood-flow velocity in respiratory-distress syndrome. Relation to the development of intraventricular hemorrhage. N Engl J Med 309:204–209
Pryds O, Greisen G, Skov LL, Friis-Hansen B (1990) Carbon dioxide-related changes in cerebral blood volume and cerebral blood flow in mechanically ventilated preterm neonates: comparison of near infrared spectrophotometry and 133Xenon clearance. Pediatr Res 27:445–449
Toet MC, Flinterman A, Laar IV, Vries JW, Bennink GB, Uiterwaal CS, Bel FV (2005) Cerebral oxygen saturation and electrical brain activity before, during, and up to 36 hours after arterial switch procedure in neonates without pre-existing brain damage: its relationship to neurodevelopmental outcome. Exp Brain Res 165:343–350
Tsuji M, Saul JP, du PA, Eichenwald E, Sobh J, Crocker R, Volpe JJ (2000) Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics 106:625–632
Volpe JJ (2001) Intracranial hemorrhage: germinal matrix-intraventricular hemorrhage of the premature infant. Neurology of the newborn. Saunders, Philadelphia, pp 428–493
de Vries LS, Rademaker KJ, Groenendaal F, Eken P, van Haastert IC, Vandertop WP, Gooskens R, Meiners LC (1998) Correlation between neonatal cranial ultrasound, MRI in infancy and neurodevelopmental outcome in infants with a large intraventricular haemorrhage with or without unilateral parenchymal involvement. Neuropediatrics 29:180–188
Wyatt JS, Cope M, Delpy DT, Wray S, Reynolds EO (1986) Quantification of cerebral oxygenation and haemodynamics in sick newborn infants by near infrared spectrophotometry. Lancet 2:1063–1066
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lemmers, P.M.A., Toet, M., van Schelven, L.J. et al. Cerebral oxygenation and cerebral oxygen extraction in the preterm infant: the impact of respiratory distress syndrome. Exp Brain Res 173, 458–467 (2006). https://doi.org/10.1007/s00221-006-0388-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0388-8