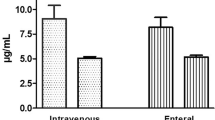
Abstract
Topical application of caffeine for the treatment of neonatal apnoea was considered in 57 low birth weight infants (<1500 g birth weight). The rationale for the study was that transdermal absorption of drugs and chemical agents has been demonstrated in neonates depending on anatomical and functional immaturity of the epidermal barrier. Considering these issues we studied the efficacy of percutaneous application of caffeine using high pressure liquid chromatography (HPLC) for evaluation of its plasma levels. 2×7.5 mg (babies <1000 g, extremely lowbirth weight [ELBW] or 2×10 mg (babies >1000 g, very low birth weight [VLBW]) of caffeine were applied transcutaneously in form of a gel to the abdominal skin (Standard dose=0.06 g of gel equivalent to 10 mg of caffeine citrate). Gestational age of our patients was 29.4±1.7 weeks, mean birth weight 1025±240 g. Mean postnatal age at beginning of treatment was 25.5±18h. Of the treated babies, 73% had serum levels in therapeutic range about 48h after the first dose of calffeine application. After 10 doses 97% of patients had serum levels in the therapeutic range. We conclude that percutaneous caffeine application is a safe and useful approach for treatment of apnoea in VLBW and ELBW infants.
Similar content being viewed by others
Abbreviations
- ELBW:
-
extremely low birth weight
- VLBW:
-
very low birth weight
References
Amato M, Straume B (1981) Iridopupillarmembran, zur Bestimmung des Gestationsalters des Frühgeborenen. Gynakol Rundsch 21:55–58
Amato M, Isenschmid M, Gambon R, Schneider H (1988) Percutaneous caffeine in the treatment of neonatal apnea. XI European Congress of Perinatal Medicine, Rome
Aranda J, Gorman W, Bergsteinsson H, Gunn T (1977) Efficacy of Caffeine in treatment of apnea in the low birth weight infant. J Pediatr 107:307–311
Ballard J, Kazmaier K, Driver M (1979) A simplified assessment of gestational age. Pediatr Res 11:374
Davis J, Abbasi S, Spitzer A, Johnson L (1986) Role of theophylline in pathogenesis of necrotizing enterocolitis. J Pediatr 2:344–346
Evans N, Rutter N, Hadgraft J, Parr G (1985) Percutaneous administration of theophylline in the preterm infant. J Pediatr 107:307–311
Fitzpatrick J, Mc Clelland MA (1983) A simple, rapid method for determining theophylline serum by HPLC. Ann Clin Biochem 70:123–126
Gorodischer R, Karplus M (1982) Pharmacokinetic aspects of caffeine in premature infants with apnea. Eur J Pharmacol 22:47–52
Harpin VA, Rutter N (1983) Barrier properties of newborn infant's skin. J Pediatr 102:419–422
Murat I, Moriette G, Blin M, Couchard M, Flouvat B, De Gamarra E, Relier JP, Dreyfus-Brisac C (1981) The efficacy of caffeine in the treatment of recurrent idiopathic apnea in premature infants. J Pediatr 99:984–989
Nachman RL, Esterly NB (1971) Increased skin permeability in preterm infants. J Pediatr 79:628–631
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Amato, M., Isenschmid, M. & Hüppi, P. Percutaneous caffeine application in the treatment of neonatal apnoea. Eur J Pediatr 150, 592–594 (1991). https://doi.org/10.1007/BF02072214
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02072214