Article Text
Abstract
Objective To evaluate the effectiveness of intermittent pulse oximetry in guiding oxygen therapy in neonates in a low-resource setting.
Design and setting Prospective validation study at three hospitals in southwest Nigeria. We performed concealed continuous pulse oximetry on participants to evaluate intermittent SpO2 monitoring.
Patients We recruited all preterm or low birthweight neonates, and all term neonates who required oxygen therapy, who were admitted to the neonatal ward(s) of the study hospitals during the study period.
Main outcome measures Proportion of time preterm/low birthweight neonates on oxygen spent within, above and below the target SpO2 range of 90%–95%; and the proportion of time term neonates and neonates not on oxygen spent within and below the target range of 90%–100%.
Results Preterm/low birthweight neonates receiving oxygen therapy (group A) spent 15.7% (95% CI 13.3 to 18.9) of time in the target SpO2 range of 90%–95%. They spent 75.0% (63.6–81.1) of time above 95%, and 2.7% (1.7–5.6) of time below 85%. Term neonates and all neonates not receiving oxygen (group B) spent 97.3% (95% CI 96.4 to 98.6) of time within the target range of 90%–100%, and 0.9% (0.3–1.4) of time below 85%. Guidelines recommended SpO2 monitoring 3 times per day for all patients, however neonates in groups A and B were monitored an average of 4.7 and 5.3 times per day, respectively.
Conclusions To better maintain SpO2 within the target range, preterm/low birthweight neonates on oxygen should have their SpO2 monitored more frequently than the current 4.7 times per day. In all other neonates, however, monitoring SpO2 5.3 times per day appears suitable.
- oxygen
- oximetry
- neonatology
- SpO2
- low-resource
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
Statistics from Altmetric.com
What is already known on this topic?
Oxygen is an essential medical therapy, but must be used judiciously in preterm and low birthweight neonates to avoid harm from retinopathy of prematurity (ROP) and bronchopulmonary dysplasia (BPD).
This requires monitoring via pulse oximetry, to keep SpO2 within a target range of 90%–95%.
In many resource-poor settings, continuous pulse oximetry is not available, necessitating the use of intermittent monitoring to guide oxygen therapy.
What this study adds?
We found that preterm and low birthweight neonates spent only 15.7% of time within the target SpO2 range of 90%–95% while receiving oxygen, spending 75% of time above 95%.
Term neonates, however, spent almost all of their time within the target range.
In preterm and low birthweight neonates receiving oxygen therapy, more frequent SpO2 monitoring could improve oxygen targeting and potentially prevent harm from ROP and BPD in resource-constrained hospitals.
Kudos summary
This study sought to evaluate the effectiveness of intermittent pulse oximetry in guiding oxygen therapy in newborns in a low-resource setting. We conducted the study in three secondary-level hospitals in southwest Nigeria, where oxygen is available for use in newborns, but continuous monitoring of oxygen saturations is not available.
We found that preterm and low birthweight newborns receiving oxygen therapy, who have a narrow oxygen saturation target range of 90%–95%, only spent 15.7% of time within the target range. They spent 75% of time with saturations above 95%, exposing them to potentially dangerous oxygen levels, which can lead to damage to their eyes and lungs. Term neonates and all neonates who were not receiving oxygen, however, spent almost all of their time (97.3%) within their wider target range of 90%–100%.
These results show that to improve oxygen targeting, preterm and low birthweight newborns who are receiving oxygen should have their oxygen saturations more frequently monitored where this is possible. They also demonstrate a need to teach health workers in resource-constrained hospitals about the dangers of using too much oxygen in these patients, and how to respond to oxygen saturation readings above and below the target range.
Introduction
Oxygen is a life-saving medical therapy for sick newborns that has been used for over 100 years.1 When used in preterm (gestational age <37 weeks) and low birthweight (birth weight <2000 g) neonates, however, it carries significant risks—most notably retinopathy of prematurity (ROP) and bronchopulmonary dysplasia (BPD).2 3 Tight control of haemoglobin oxygen saturation (SpO2) through use of pulse oximetry can prevent harm.2 4
The principal and most studied complication of hyperoxia in preterm neonates is ROP. In high-income countries (HICs), ROP is now uncommon due to effective SpO2 control in at-risk neonates.5 However, its prevalence is increasing in low-income and middle-income countries (LMICs) as neonatal preterm survival increases and oxygen is used excessively. This has been labelled the ‘third epidemic’ of ROP, and is thought to be responsible for 20 000 new cases of blindness annually.6 7
While there continues to be some debate about the optimal SpO2 range for preterm and low birthweight neonates, it is thought to lie between approximately 90% and 95%,2 4 with WHO recommending 88%–95%.8 However, it is challenging to maintain SpO2 of preterm and low birthweight infants within these target ranges, and they often spend large proportions of time outside.9–11 SpO2 targeting is particularly challenging in LMICs. Despite improvements in recent years, oxygen practices in many LMICs remain poor and pulse oximetry is rarely used to guide therapy. Hospital surveys consistently report very low availability of pulse oximeters for paediatric and neonatal use (typically <10%) and low staff awareness of how pulse oximetry should be used in children and newborns.12–16
In these settings, continuous SpO2 monitoring is often not possible and intermittent monitoring is increasingly used to guide oxygen therapy. However, it is unclear how effective it is in guiding oxygen therapy, particularly in neonates at risk of ROP and BPD.
This study seeks to evaluate the effectiveness of intermittent SpO2 monitoring in guiding oxygen therapy in neonates in a low-resource setting.
Methodology
Study design
We conducted a prospective validation study at three secondary-level hospitals in southwest Nigeria, between June 2017 and March 2018. We evaluated the effectiveness of intermittent pulse oximetry monitoring (the current standard of care at all study hospitals) in guiding oxygen therapy in neonates by performing concealed continuous pulse oximetry on participants during routine care.
Participants
We recruited all preterm or low birthweight neonates, and all term neonates who required oxygen therapy, who were admitted to the neonatal ward(s) of the study hospitals during the study period. Inclusion and exclusion criteria are detailed in table 1.
Inclusion and exclusion criteria for participants at all study hospitals
Procedures
Intermittent pulse oximetry using Lifebox pulse oximeters (Acare Technology, Taiwan) was a standard practice in all hospitals. We did not provide additional refresher training or reminders, as we intended to evaluate routine care. Staff had previously received training and supportive supervision on pulse oximetry and the oxygen protocol in November 2015.16 17 The oxygen protocol recommended using pulse oximetry for every neonate admitted to the nursery, and commencement of oxygen therapy if any SpO2 reading was <90%. Pulse oximeter probes were attached to neonates’ hands or feet and nurses recorded the SpO2 reading in the patient’s clinical observation chart after an adequate plethysmographic waveform was observed and the SpO2 reading was stable (typically within 1–2 min).16 The protocol recommended checking the SpO2 on admission, within 15 min of any change in oxygen flow rate, and at least once per shift (3 times per day), or more frequently for neonates with severe respiratory distress or signs of deterioration. It recommended aiming for SpO2 90%–95% for preterm or low birthweight neonates receiving oxygen therapy and SpO2 ≥90% for other neonates. Monitoring data had shown consistently high use of pulse oximetry for neonates on admission (>90%) and strong adoption into routine care practices.16
Data collection at each site was performed by a dedicated study nurse or PJBW, under the supervision of the project coordinator and co-principal investigators. We commenced concealed continuous SpO2 monitoring using Masimo Radical-7 CO-Oximeters (Masimo, Irvine, California, USA) after admission to the neonatal ward (or after commencement of oxygen therapy for term neonates), and continued it until 48 hours after oxygen was ceased, or to a maximum of 5 days (120 hours) for each patient. We concealed the visual display and disabled normal upper and lower limit alarms of the Masimo continuous oximeters so SpO2 readings could not be determined by staff or study investigators. However, to prevent avoidable harm from severe hypoxaemia occurring, we set a lower audible SpO2 alarm limit at 80%, alerting nurses to potentially dangerously low SpO2 and prompting clinical review. We also ensured the plethysmographic waveform was visible to permit us to evaluate the accuracy of SpO2 readings. We collected data from the first study hospital (SH1) between 22 June 2017 and 31 July 2017, and the second and third study hospitals (SH2, SH3) between 29 November 2017 and 20 March 2018.
We downloaded SpO2 data using Profox oximetry software (Profox Associates, Coral Springs, Florida, USA), yielding .xlsx files, which we opened using Stata V.14.0 (StataCorp, College Station, Texas, USA) to enable cleaning and analysis.
Outcomes
The primary outcome for this study was the proportion of time preterm/low birthweight neonates spent in the target SpO2 range (90%–95%) while receiving oxygen therapy. Secondary outcomes were the proportion of time spent by 1) preterm/low birthweight neonates and 2) term neonates:
Below safe levels (SpO2 <85%);
Within safe range (SpO2 85%–95% for preterm/low birthweight neonates on oxygen; 85%–100% for term neonates, or any neonates not on oxygen);
Within target range (SpO2 90%–95% for preterm/low birthweight neonates on oxygen; 90%–100% for term neonates, or any neonates not on oxygen);
Above safe levels (SpO2 >95% for preterm/low birthweight neonates on oxygen).
Analysis
We stratified participants into those with a target SpO2 range of 90%–95% (preterm/low birthweight neonates on oxygen) and those with a target range of 90%–100% (all other neonates). We then reported the primary and secondary outcomes as median values with 95% CIs, as data were not normally distributed. We used Stata V.14.0 to conduct all data cleaning and analysis.
Ethical aspects
This study was conducted in accordance with the Australian National Statement on Ethical Conduct in Human Research. We obtained written informed consent from a parent/guardian of all participants before enrolment in this study.
Results
We recruited a total of 86 eligible neonates for this study: 41 from SH1, 26 from SH2 and 19 from SH3. Fifty-one participants were either preterm or low birth weight (19 at SH1, 17 at SH2, 15 at SH3), and 70 received oxygen during their admission (35 at SH1, 26 at SH2, 9 at SH3). Patient characteristics are detailed in table 2.
Patient characteristics for all participants at all study hospitals
We monitored participants’ SpO2 for a total of 5552 hours (231.3 patient-days). Of this, 4000 hours (166.7 days, 72.1% of total recording time) were for preterm/low birthweight neonates, including 1766 hours (73.6 days; 31.8%) while receiving oxygen; 1551 hours (64.6 days, 27.9%) were for term neonates.
Frequency of monitoring
During the study period, nurses recorded 1147 intermittent SpO2 readings on participants (mean 5.0 readings per patient per day) during routine care: 717 on preterm/low birthweight neonates (mean: 4.3 readings per patient per day; 4.7 while on oxygen; 4.2 while not on oxygen); 430 oximetry readings on term neonates (mean: 6.7 readings). Patients with a target SpO2 range of 90%–95% had their SpO2 monitored 4.7 times per day; those with a target range of 90%–100% were monitored 5.3 times per day (both mean values).
Time within the target range
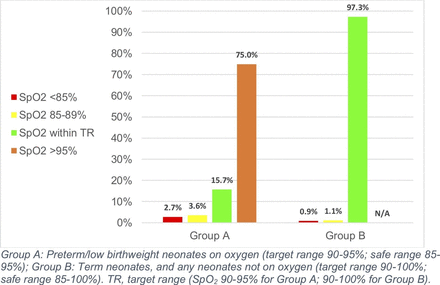
Preterm and low birthweight neonates on oxygen spent only 15.7% (95% CI 13.3 to 18.9) of time within the target SpO2 range of 90%–95%. These neonates spent 75.0% of their time (63.6–81.1) with SpO2 >95%, and 2.7% (1.7–5.6) of their time with SpO2 <85% (table 3, figure 1). Term neonates, and all neonates not on oxygen, however, spent 97.3% (95% CI 96.4 to 98.7) of their time within the target range of 90%–100%, and only 0.9% (0.3–1.4) of their time with SpO2 below 85%. Analysing both groups together, participants spent on average 92.5% (95% CI 78.5 to 96.3) of time within the target range, and only 1.5% (1.0–2.3) of time with SpO2 <85%.
Median proportion of time participants spent within and outside of target SpO2 ranges during the study period.
Proportion of time participants spent within and outside target and safe SpO2 ranges during study period
Compared with concealed continuous SpO2 monitoring, nurses’ oximetry records detected more time below the target range and less time above the target range (table 4). This was most evident in preterm infants on oxygen therapy.
Proportion of time participants spent within and outside target and safe ranges, comparing data from concealed continuous SpO2 monitoring and nurses’ clinical records from intermittent monitoring
Discussion
Our findings suggest that current oximetry and oxygen practices in the study hospitals are appropriately maintaining SpO2 in the target range for term neonates, but are not adequately restricting oxygen therapy for preterm and low birthweight neonates. While term neonates spent 97% of time in the target range (90%–100%), preterm/low birthweight neonates spent 15.7% of time in the target range (90%–95%) while receiving oxygen. Neonates spent very little (<2%) time below safe levels (<85%), suggesting that oxygen therapy is being used effectively to prevent and treat hypoxia and its potentially lethal sequelae.18 However, preterm neonates on oxygen therapy spent 75% of time above the target range (>95%). We did not measure ROP or BPD in our study, but the link between iatrogenic hyperoxia and these conditions is well-established4 19–21 and excessive oxygen administration in the study hospitals therefore likely put these neonates at risk of harm.
Nurses’ intermittent SpO2 recordings tended to overestimate the time spent below the target range and underestimate the time spent above the target range. This may indicate that nurses more actively look for (and act on) hypoxia than hyperoxia—a hypothesis supported by feedback from nurses at SH1 and previous studies.22 23 This has implications for auditing and designing oxygen systems in low-resource hospitals, where relying on recorded SpO2 readings may overestimate the time spent under target range, and underestimate the time spent above.
The Lifebox pulse oximeters used by nurses in this study were developed for low-resource settings by WHO and the World Federation of Societies for Anaesthesiologists, with rated accuracy of ±2%.24 25 They have been validated for use in children and neonates, and give comparable readings with leading commercial oximetry brands.24 26
Existing evidence shows that even in resource-rich settings, it is difficult to control SpO2 accurately in preterm and low birthweight neonates. Data from large oxygen-targeting trials among extremely preterm neonates in HICs found that neonates spent 41%–67% of time outside the target range.9–11 27 28 Oxygen targeting in our study was expectedly poorer than these studies, which were conducted in well-resourced settings using continuous pulse oximetry monitoring by dedicated neonatal intensive care nurses. Data from Kenya involving intermittent SpO2 monitoring found that in the first 24 hours of life, only 6.7% of preterm/low birthweight neonates remained consistently within the target SpO2 range, and more than half (53%) had more SpO2 readings outside the target range than within.29
Common oxygen-related and oximetry-related challenges facing clinicians caring for neonates in resource-poor environments include:
Decreased capacity to detect hypoxic or hyperoxic episodes, which can increase the risk of ROP30;
Nurse:patient ratios as low as 1–2 nurses to 40–50 neonates27;
Suboptimal staff education in oxygen administration to preterm/low birthweight neonates;
Tendency to favour hyperoxia over hypoxia. This observation is consistent with previous studies, and is thought to be related to the innocuous clinical presentation of hyperoxia compared with hypoxia.9 11 27 28
Improving oximetry and oxygen practices in a low-resource environment
Improved access to oxygen therapy globally has been associated with decreased neonatal mortality in many LMICs (particularly MICs).31 However, it has also fuelled a rise in the prevalence of ROP.6 32 33 Many hospitals now face challenges in the safe use of oxygen in preterm/low birthweight neonates. Our study shows that while pulse oximetry is an essential tool for promoting safe oxygen use, additional measures may need to be introduced for preterm and low birthweight neonates, who are most at risk of harm.
Potential avenues for improvement relate to the frequency of SpO2 monitoring, and adequate resource-provision and staffing of neonatal wards. The most financially feasible of these is likely frequency of SpO2 monitoring. As this study shows, checking SpO2 of neonates with a target range of 90%–100% 5.3 times per day is sufficient to keep SpO2 within the desired range. However, in preterm/low birthweight neonates receiving oxygen, monitoring SpO2 more frequently than the current 4.7 times per day could lead to improved SpO2 control. Informal feedback from nurses at SH1 suggested that monitoring these neonates’ SpO2 up to 12 times per day could be feasible, particularly as this change would only involve a minority of neonates. Structural changes such as designated high-dependency zones in neonatal wards with greater nursing resources could help achieve this. All changes to practice should, however, be determined by what is feasible and appropriate in each individual clinical setting.
Recently, automated closed-loop systems have been shown in high-income settings to enable superior SpO2 control in preterm/low birthweight neonates.34–36 Further investigation is needed to ensure quality and safety of such systems, but application of this technology to simple oxygen systems in resource-constrained settings remains an exciting possibility.
Finally, many commonly used oxygen and oximetry guidelines, including those by WHO, do not make specific reference to how intermittent pulse oximetry should be used in neonates.8 Given the increasing use of this monitoring method in LMICs, there is future potential to include clearer guidance on its optimal use in neonates, particularly those born preterm or with low birth weight.
Limitations
This study has several limitations. First, the number of neonates enrolled in this study was low and involved only three hospitals. Furthermore, few participants were very/extremely preterm or very low/extremely low birth weight, the demographic most at risk of hyperoxic injury. Additional data in this patient group from low-income settings would be helpful to guide future oxygen practices. The study hospitals were also participating in an oxygen improvement project, and had already improved their oxygen practices substantially prior to this study. In the 2 years prior to this study, all three hospitals had adopted pulse oximetry into routine practice, and installed oxygen delivery systems that enabled them to more easily and accurately provide oxygen to patients (including 0-2 LPM flowmeters, nasal prongs, oxygen concentrators and reliable power supply).16 17 Unpublished data show that the introduction of pulse oximetry did improve the quality of oxygen care in our study hospitals (personal correspondence, Dr Hamish Graham, January 2019). As such, these hospitals represent relatively good oxygen and pulse oximetry practices compared with most similarly resourced hospitals.
Conclusions
In this study, we found that in preterm and low birthweight neonates receiving oxygen therapy, monitoring SpO2 intermittently an average of 4.7 times per day using a simple decision-making algorithm was only sufficient to keep SpO2 within the target range of 90%–95% for a small proportion of time. However, the same procedure enabled nurses to adequately maintain all other neonates’ SpO2 within a wider target range of 90%–100%. Preterm and low birthweight neonates on oxygen therapy spent 75% of time in hyperoxia, indicating a need to teach health workers to be alert to the dangers of hyperoxia and the safe upper threshold in these infants, and to enable them to monitor SpO2 frequently. Either intermittent or continuous pulse oximetry must be associated with adequate awareness and responses to avoid dangerous hypoxaemia or hyperoxia.
References
Footnotes
Contributors PJBW, HG, TD, KT and AGF conceived and designed the study. PJBW, HG, AAB, AIA, ROO, OAO, IVO and AGF participated in project implementation. PJW and AAB were responsible for data collection. PJBW, HG and AAB contributed to data analysis and interpretation of results. PJBW drafted the manuscript. HG, TD and AGF provided substantial comments to the writing of the manuscript. All authors read and approved the final manuscript.
Funding The funders of the study include the Bill and Melinda Gates Foundation (financial support for the Nigeria Oxygen Implementation Project, in which this study is nested; OPP1123577), Masimo Corporation (provision of 10 Radical-7 CO-Oximeters, probes, and one Windows laptop computer) and the Murdoch Children’s Research Institute ($A500 scholarship to support PJBW’s involvement). All authors had full access to all the data in the study, and had final responsibility for the decision to submit for publication.
Disclaimer The funders played no role in study design, data collection, data analysis, data interpretation or writing of this report.
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval This study obtained approval from the ethics committees at the University of Melbourne (Ethics ID 1748914), Monash University (Project Number 9398) and the University College Hospital/University of Ibadan (Ethics Committee Assigned Number UI/EC/16/0413).
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available on reasonable request.