Article Text
Abstract
Objectives To investigate the emergence of biological rhythms in the first months of life in human infants, by measuring age-related changes in core body temperature during night-time sleep, hormones (cortisol and 6-sulfatoxymelatonin) and the expression of a clock-controlled gene H3f3b in oral epithelial cells.
Design Observational longitudinal study.
Setting We measured overnight core body temperature, actigraphy, day–night urinary cortisol and 6-sulfatoxymelatonin, as well as circadian gene expression, in infants at home from March 2007 to July 2008 in Leicester.
Participants We recruited 35 healthy Caucasian infants who were born at term. They were monitored from 6 to 18 weeks of age.
Results At 8 weeks of age the day–night rhythm of cortisol secretion was the first to appear followed by 6-sulfatoxymelatonin 1 week later; at the same time that night-time sleep was established. At 10 weeks, the maximum fall in deep body temperature occurred with the onset of night-time sleep, followed at 11 weeks by the rhythmical expression of the H3f3b gene.
Conclusions In human infants, there is a clear sequential pattern for the emergence of diurnal biological rhythms between 6 and 18 weeks of postnatal age, led by the secretion of cortisol and linked with the establishment of consolidated night-time sleep. It is likely that this represents part of a maturation and adaption process as infants gain equilibrium with their external environment after birth.
- Sleep
- Fetal Medicine
- SIDS
Statistics from Altmetric.com
What is already known on this topic?
-
All organisms have biological rhythms.
-
In mammals, these rhythms mature in very early postnatal life.
-
Most data are in animals, not humans.
What this study adds?
-
The human infant develops distinct biological rhythms in sequence.
-
Cortisol appears to initiate and control this process.
-
Infant biological rhythms are closely linked to sleep.
Introduction
In utero, biological rhythms in the growing fetus are largely under maternal control.1 This maternal ‘physiological protection’ is relinquished at birth as the newborn begins to establish its own physiological and physical independence. Along with the obvious cardiorespiratory changes at birth, equally critical physiological adaptation takes place. This adaptation continues for the next 4–6 months2 ,3 as the infant consolidates sleep. Wailoo et al4 have documented the age-related changes in deep body temperature in infants during the early postnatal months and have identified some of the factors which may influence this development.5 ,6
In an extension of previous work,4 the main objective of our study was to ascertain, for normal infants, the development of biological rhythms and circadian gene expression. In this study, longitudinal measurements were made in the home on infants mainly during night-time sleep, while checking the expression of the circadian histone gene H3f3b (see below) in buccal swabs, measuring cortisol and 6-sulfatoxymelatonin (MT6s; a major melatonin metabolite) secretion in urine and monitoring changes in core body temperature at night.
Ultimately, we will establish the baseline of normal circadian rhythm development and be able to elucidate the phased integral physiological adaptation and maturation that is known to occur. In addition, we will identify biological clock mechanisms which are laid down antenatally and assembled simultaneously during that period of postnatal development.
Methods
Selection of participants
We approached parents of singleton, full-term normal birthweight infants, on the postnatal ward at Leicester Royal Infirmary (UK), for an expression of interest, and recruited from March 2007 to July 2008.
We then visited parents once between weeks 4 and 6, to explain the study and obtain written informed consent. Monitoring visits took place at home from 6 weeks to a maximum of 18 weeks on a fortnightly basis. Approval was given by the Local Research Ethics Committee.
Body temperature measurements
Three temperature probes were sited: a soft rectal probe inserted into the baby's rectum 5 cm from anal margin, a skin probe on the right shin and third, a probe to record the room temperature placed 30 cm from the cot. All probes were attached to a portable battery operated data logger (Squirrel 1201, 1202 models from Grant instruments Cambridge, UK) and were set to record minute by minute throughout the night, beginning approximately 1 h before sleep and ending with final morning waking.
After the first recording, monitoring continued at fortnightly intervals until a mature temperature pattern during night-time sleep could be demonstrated, that is, a fall of deep body temperature below 36.5°C within 4 h of sleep onset. At the same time we measured sleep activity, collected urine samples and buccal smears as outlined below.
Sleep activity
No adjustments were made to the routine care or infant sleep environment. We fitted a mini Actiwatch (Cambridge Neurotechnology Limited, Cambridge, UK) around the ankle of each infant, which measures movement to obtain an approximation of rest and activity overnight as a proxy for sleep and wakefulness. Sleep onset was defined as the first period after recorded bedtime with at least 10 min of consecutive recorded immobile data (as per sleep-wake scoring algorithm). Mothers also kept a sleep diary.
Urine collection
Parents collected urine samples from their infant at midday and midnight for cortisol and MT6s estimation7 by fitting a Hollister U bag or pad (Uricol, Newcastle, UK) within 30 min of desired collection time. Samples were collected on two consecutive days, on a weekly basis. The MT6s urinary metabolite has a strong correlation with urinary/serum melatonin.8 Samples were frozen within 4 h and analysed by radioactive immunoassay (RIA; Stockgrand, University of Surrey, UK). We applied a standard correction for renal excretion.9
Collection of buccal swabs for circadian gene analysis
Each week parents took infant buccal swabs every 6 h over a 2-day period, using foam tipped Catch-all swabs (Epicentre, Madison, Wisconsin, USA). Swabs were preserved in RNA Later (Ribose Nucleic Acid Later; Ambion, USA) and frozen at −20°C. The samples were then transported in dry ice and stored at −80°C until analysis.
Laboratory analysis
Total RNA was extracted using the Qiagen RNAeasy mini kit. Yields varied from 20 pg/µL to 1 ng/µL. We performed cDNA synthesis using the Transcriptor First Strand cDNA Synthesis kit (Roche Applied Science). Gene expression was measured by reverse transcription real-time quantitative PCR using the Opticon 1 (MJResearch) thermocycler. Swabs from infants rendered a very low yield of RNA, hence it was necessary to measure as an indicator of clock function, the rhythmical transcript of the gene Histone 3 family 3b (H3f3b).10 This produces a protein that makes up chromatin in the nucleus of eukaryotic cells and is expressed in a circadian fashion.11 The house-keeping gene glyceraldehyde 3-phosphate dehydrogenase (Gapdh) was used as an internal control of amplification (further detail is provided in the online data supplement).
Data analysis: definition of rhythm development
We compiled the temperature data in 30-min intervals. A mature temperature rhythm was achieved at the age when maximum drop in core body temperature within the first 4 h of sleep onset occurred, as has been described previously.4 The actigraphy data were analysed using commercial software (Cambridge Neurotechnology, UK). We recorded the week of maximum sleep efficiency (percentage of time in bed that infant was asleep during night only). A diurnal rhythm for cortisol was achieved when more than 50% of total hormone was secreted during the day than at night, whereas the opposite was used for MT6s (ie, more secreted in the night than in day).12 The week circadian gene expression reached the maximum value or amplitude was selected as week of maturation of rhythm.
All main data outcomes had a normal distribution apart from MT6s and cortisol concentrations. These were logarithm transformed. We compared all data using mean values, SD and parametric tests. For circadian gene expression, a sine wave curve was fitted using the mean threshold (Ct) value to assess the amplitude and phase of diurnal variation in peripheral gene expression. Analysis was undertaken using SPSS (V.16) and STATA (V.12) packages. The level for statistically significant results was set to 5%.
Results
We recruited 35 (15 male and 20 female) Caucasian infants, of which 18 (51%) were exclusively breast fed, 7 (20%) were breast fed up to 6 weeks of age and then formula fed, 4 (11%) were fed a mixture of breast and formula feed and 6 (17%) were exclusively formula fed. The mean±SD gestational age was 39.5±1.4 weeks and average birth weight was 3515±644 g (see table 1).
Description of participating infants and families
Core body temperature measurements
One hundred and thirty-six temperature recordings were analysed, from 35 infants between 6 and 18 weeks, with each infant having a mean of 4.2 measurements (range 1–6). Eleven recordings were inadequate due to probe dislodgement or equipment failure.
The infants demonstrated an age-related, longitudinal change in core body temperature during night-time sleep. The mean core temperature (SD) just prior to sleep onset was 37.2°C (±0.27°C) for all ages. The core temperature fell within 4 h of sleep onset (denoted as time 00:00 h) and the depth of this fall increased with age (figure 1). The core temperature remained low throughout the night, with a gradual rise in temperature as the infant approached waking. The lowest value mean (SD) was 36.4°C (±0.43) at 10.8 weeks. There was no further fall with increasing age.
Mean core body temperature values for healthy term infants at 30-min intervals from 1 h prior to sleep onset to 8 h after sleep onset. Each infant was monitored at fortnightly intervals for one night only. The graph shows mean values grouped into intervals of 5–9 weeks, 10–12 weeks and 13–18 weeks with repeated measures. Error bars are excluded for clarity.
Cortisol and MT6s secretion
Four hundred and forty-six urine samples were analysed for cortisol estimation (22 insufficient samples) and 464 samples (four insufficient samples) for MT6s from 34 infants. Samples were collected very close to target times, on average at 23:47 h. for midnight and 11:50 h for midday collection.
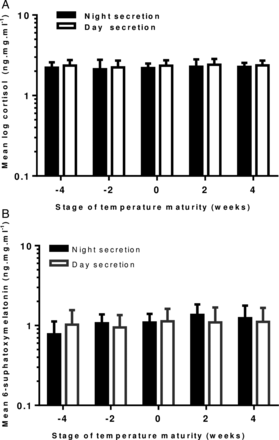
Day-time cortisol secretion (SD) accounted for 60 (±21) % of total cortisol secretion. There was no overall increase or decrease in cortisol secretion with age, either in the day or in the night.
Night-time secretion of MT6s (SD) was 51% (±21) of total, across all ages, which increased with age, week by week by 21% (p<0.001; 95% CI 13% to 29%). There was no overall change in day-time secretion.
We calculated mean cortisol and MT6s levels at fortnightly intervals and grouped these in weeks prior to and after onset of mature temperature. The difference between night and day values did not achieve statistical significance (figures 2A, B).
(A) Changes in day and night urinary cortisol concentration 4 weeks prior and 4 weeks after the development of a mature temperature pattern in healthy term infants. (n=14 at −4 weeks, n=21 at −2 weeks, n=17 at 0 weeks, n=18 at 2 weeks and n=7 at 4 weeks). Error bars=1 SEM. (B) Graph showing changes in day and night urinary concentration of 6-sulfoxymelatonin four weeks prior and 4 weeks after the development of a mature temperature pattern in healthy term infants. (n=16 at −4 weeks, n=23 at −2 weeks, n=25 at 0 weeks, n=20 at 2 weeks and n=7 at 4 weeks). Error bars=1 SEM.
Infant sleep
We analysed 135 actigraphy readings from 35 infants with three readings lost due to equipment failure. Actigraphy (actual sleep time 20:42) confirmed parental reported mean bed time of 20:40 (p=0.903). The mean (SD) total sleep duration for all infants was 7 h (±1.8) per night, ranging from 3.5 to 10.5 h per night. The mean time (SD) taken to fall asleep once put to bed, that is, the sleep latency, was 15 (±11) min. The proportion of time (SD) infants were asleep, while in bed, that is, the sleep efficiency, was 75.3 (±10.9) %.
Circadian gene expression
A total of 22 infants had 599 buccal swab samples analysed from a total of 999 samples. One family had no refrigeration, another declined swab collection. Other samples were discarded due to illegible labelling. The remaining 10 infants did not have sufficient data (less than four consecutive six hourly samples/week). Infants with no or insufficient samples were more likely to be from low social classes (x2=3.998; df=1; p=0.046). No difference was observed for gender (p=0.762), smoking status (p=0.458), birth weight (p=0.487), gestation (p=0.695) and maternal age (p=0.794).
Linear modelling analysis showed a highly significant diurnal rhythm (sinusoidal pattern with intra/interinfant variation in amplitude and phase) with a 24-hour period (p<0.001) and an overall estimated amplitude of 0.0126 (on same scale as H3f3b/Glyceraldehyde-3-phosphate levels). The variation in the mean level of expression between the infants was also highly significant (p<0.001). The overall estimated time of day of maximum expression was 21:39 h. A representative circadian gene plot for one infant is shown in figure 3. As the infant got older the closeness of fit of pattern to sinusoidal curve emerged, although this pattern was not universally maintained.
The quantitative analysis of the expression of a clock-controlled gene H3f3b in oral epithelial cells from infant with identity number 16 003, shown as a ratio of H3f3b/Gapdh. RNA was extracted and converted to cDNA by real-time PCR. Each sample was amplified in triplicate and the H3f3b was then normalised to Gapdh gene expression. Mean Ct values were calculated. Weekly measurements were made over two consecutive days at approximately 18:00 h and 06:00 h. Sinusoidal plots are not shown. There are no data for week 9 due to insufficient samples.
Timing of biological rhythms in relation to sleep
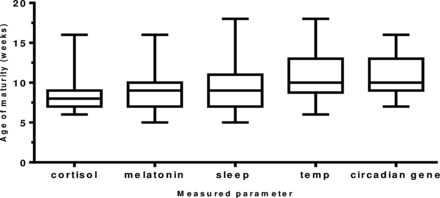
The maturation of the biological rhythms occurred in the following sequence. Maturation of the cortisol rhythm (n=35 infants) occurred first at 8.2 weeks of age. The melatonin metabolite MT6s (n=34 infants) and sleep efficiency (n=35 infants) rhythms occurred next at 9.1 and 9.4 weeks of age (p=0.6524). Temperature maturation (n=34 infants) occurred at 10.8 weeks (p=0.053) and last of all, maximum peripheral circadian gene expression (n=21 infants) at 10.9 weeks (0.932; figure 4).
The age (in weeks) of maturation of the development of circadian rhythms in healthy term infants. A diurnal rhythm for cortisol and melatonin metabolite (6-sulfatoxymelatonin) was achieved when >50% of total urinary secretion was achieved in day for cortisol and in the night for melatonin metabolite. Age of maturity of core body temperature was achieved when minimum temperature of 36.4°C occurred within first 4 h of sleep onset; for sleep, maturity was denoted as the week maximum sleep efficiency was obtained and for the peripheral circadian gene H3f3b the week maximum amplitude occurred. Number of infants: cortisol (n=35), melatonin metabolite (n=34), sleep (n=35), temperature (n=35) and H3f3b gene expression (n=22).
Discussion
The findings of this study show that human infants adapt and mature physiologically during a process: integrated and ordered in an age-related manner. There is an emergence of a series of discreet physiological functions with that of cortisol in the lead at 8 weeks of age, followed a week later by the rhythmical secretion of MT6s (melatonin), the chemical messenger of the ‘clock’. Then follows the establishment of maximum sleep efficiency also at 9 weeks and finally a mature deep body temperature rhythm at nearly 11 weeks together with peripheral Histone gene expression, that is, H3f3b.
This sequential pattern of the age-dependent development of individual physiological functions has similarly been described in the rat, where a cortisol response was present first at postnatal day 14, but a full circadian rhythm not established until later.13 Thermoregulatory control was seen within 19 days,14 sleep maturation with a decrease in rapid eye movement (REM) sleep observed from days 14 to 2715 and maximum melatonin retinal uptake at day 20.16
This appears to give cortisol a pivotal role as an instructor of independent physiological function in the newborn, and an entrainer of an integrated biological clock which enables the human infant to adjust to its new environment as Dickmeis17 has shown in other animals. Of interest, exogenous glucocorticoids are known to synchronise circadian gene expression in diverse cell types and tissues in culture,18 ,19 possibly via the glucocorticoid response elements located in the DNA regulatory regions of central clock genes.20 Cortisol production in utero and subsequent early maturation of hypothalamic pituitary adrenal axis suggest that there is a prenatal regulatory role21 that extends into life postnatally.2 ,22 The emergence of the rhythmical secretion of melatonin soon after that of cortisol suggests a temporal relationship and may be mediated by the steroid-dependent transition of the enzyme, which produces the melatonin precursor serotonin.23 The timing of the rhythms may vary with studies finding a melatonin morning peak after 3 months of age24 and a declining morning cortisol level from 6 weeks onwards.25 The evening peak of melatonin secretion shown in this study, however, coincides with the development of a consolidated day–night sleep–wake cycle.
It is not surprising that sleep efficiency improves with evening secretion of melatonin with its sleep promoting and phase shifting properties26 emphasising the importance of this relationship in the first months of life.27 However, as actigraphy has yet to be validated in infants28 and has a low specificity (39%) for wakefulness after sleep onset,29 alternative methods of measurement of sleep should be considered in future studies to characterise sleep more accurately.
With maximum sleep efficiency, we saw a characteristic fall in core body temperature at sleep onset. The likelihood is that core body temperature fell as a direct result of sleep, as observed with other physiological functions such as heart rate and that sleep was not initiated by a fall in core temperature as suggested by Kräuchi et al.30 Whether more accurate measures such as the assessment of rapid eye movement31 by polysomnography would be a more sensitive marker of early sleep maturation32 is open to question.
All rhythms are thought to be controlled by the body's master clock, the suprachiasmatic nucleus, the elements of which are laid down in utero. These are gradually assembled postnatally under neuronal and hormonal control (eg, cortisol and melatonin), leading to the expression of regulatory genes, whose role has not been previously described in human infants. The genetic basis for circadian rhythmicity has been mainly from animal work.33 In spite of methodological and practical difficulties, such investigation can be undertaken on human infants. Tissues such as epithelial cells are accessible and provide genetic material from which the maturation of the clock can be monitored.10
The distinct sinusoidal pattern of gene expression in H3f3b transcripts in buccal mucosa occurred at 11 weeks, with a mean peak of 21:39 h. Although we could not accurately explain how the pattern of peripheral gene expression relates to key clock genes due to minimal RNA extraction and the wide intrainfant and interinfant variations with age; we may nonetheless speculate that gene expression in peripheral organs (which is tissue specific) may depend on prior rhythm establishment in physiology and behaviour.
Limitations included the time commitment from families and the sensitivity and specificity of methods used. Different methods would have allowed more precise elucidation of the rhythms, distinguishing shorter ultradian from 24 h rhythms. However, other work has had similar constraints on frequency of measures and methods.24 ,25 ,34 ,35 The main strength of this study was that mothers acted as the primary research assistant, thereby enabling it to be conducted in the home, reflecting physiological maturity in a natural environment.
In conclusion, fetal rhythms continue to develop postnatally. In infants, delays in circadian rhythm development may be linked to infant mortality such as sudden infant death syndrome.36 Emerging paediatric research is highlighting the contribution of circadian rhythm disruption to morbidity and mortality37 ,38 and this is being incorporated into principles of clinical practice.39
Acknowledgments
We thank Dr Benita Middleton for assistance with hormone assays and Professor John Thompson for invaluable statistical advice and support.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online supplement
Footnotes
-
Contributors DJ collected the data, carried out initial analyses, drafted and revised the manuscript and approved the final manuscript as submitted. NWC contributed to study design, supervised data analyses, reviewed and revised manuscript and approved final manuscript as submitted. MES conducted gene expression analyses, reviewed and revised the manuscript and approved the final manuscript as submitted. NAT supervised initial analyses and conducted secondary analyses, critically reviewed and revised manuscript and approved final manuscript as submitted. ER contributed to study design, supervised gene expression analyses, reviewed and revised manuscript and approved final manuscript as submitted. SAP, MES and WPW critically appraised, reviewed and revised manuscript and approved final manuscript as submitted. MW conceptualised and designed the study, supervised data collection and initial analyses, reviewed and revised manuscript and approved final manuscript as submitted.
-
Funding This study was supported by a grant from Babes in Arms and Leicester City West Primary Care Trust.
-
Competing interests None.
-
Patient consent Obtained.
-
Ethics approval Local Research Ethics and Committee (LREC) Ref 06/Q2405/27.
-
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- Fantoms