Article Text
Abstract
AIMS To compare the ultrasound (US) evolution and neurodevelopmental outcome of infants with localised (grade II) and extensive (grade III) cystic periventricular leucomalacia (c-PVL).
METHODS Over a nine year period, c-PVL was diagnosed in 96/3451 (2.8%) infants in two hospital cohorts. Eighteen were excluded from the study. Thirty nine infants with grade II PVL were compared with 39 infants with grade III PVL.
RESULTS The two populations were comparable for gestational age and birth weight. In infants with grade II PVL, cysts were noted to develop more often after the first month of life (53%) in contrast with grade III PVL (22%) (odds ratio (OR) 3.81 (95% confidence interval (CI) 1.19 to 12.63)). Cysts were also more often unilateral in grade II (54%) than in grade III PVL (0%) (OR indefinite; RR 3.17 (95% CI 2.16 to 4.64)). At 40 weeks postmenstrual age (PMA), cysts were no longer seen on US in 13/38 infants with grade II PVL, with ventriculomegaly being the only visible sequel in nine cases. In grade III PVL, cysts were still present in 25 of the 27 surviving infants. Nine infants with grade II PVL were free of motor sequelae at follow up compared with one infant with grade III PVL (OR 8.07 (95% CI 0.92 to 181.66)). Twenty two out of 29 children with grade II PVL who developed cerebral palsy achieved independent walking compared with 3/26 with grade III PVL (OR 75 (95% CI 11.4 to 662)).
CONCLUSIONS In the cohort studied, 50% of the infants with c-PVL had a more localised form (grade II). In grade II PVL, the cysts developed beyond the first month of life in more than half of the cases and were often no longer visible, on US, at 40 weeks PMA. In order not to miss this diagnosis, sequential US should also be performed beyond the first month of life. Mild ventriculomegaly noted at term can sometimes be due to grade II c-PVL. Cerebral palsy was slightly less common and tended to be less severe in infants with grade II PVL than in those with grade III PVL.
- periventricular leucomalacia
- ultrasound
- neurodevelopmental outcome
- ventriculomegaly
- cerebral palsy
Statistics from Altmetric.com
The diagnosis of periventricular leucomalacia (PVL) was initially only made when areas of increased echogenicity on cranial ultrasound (US) evolved into cystic lesions.1 ,2Subsequently, prolonged “flares” were considered to be a milder degree of PVL. More recently, attention has been paid to ventriculomegaly, and it was suggested that ventriculomegaly on its own could be a marker of white matter disease.3 ,4 Cystic PVL (c-PVL), referred to in the literature, is usually extensive c-PVL (grade III according to de Vries et al 5). The presence of smaller and more localised cysts (grade II according to de Vries et al 5) is less commonly reported. Fawer and Calame6 were the first to report the diagnosis of localised c-PVL and the subsequent neurological development in a small group of preterm infants. Evolution of the US lesions in PVL grade II has, however, not yet been reported in detail.
Initial follow up studies7-10 reported the outcome of children with c-PVL, irrespective of the site, type, and extent of the lesions. Subsequent studies11-15 did, however, describe c-PVL in more detail, taking the site and extent of the lesion into account and relating these findings to the neurodevelopmental outcome. The data of most of these studies were recently analysed together by Holling and Leviton,16 who reported a prevalence of cerebral palsy in only 35% of infants with “small lesions”.
The aim of this study was to compare the US evolution and the neurodevelopmental outcome of infants with grade II and III PVL.
Methods
Between 1990 and 1998 all preterm infants (n = 3451) of 32 weeks gestation or less, admitted to the level III neonatal intensive care units of Lille and Utrecht, were enrolled in a prospective cranial ultrasound (US) study.
CRANIAL US ASSESSMENT
Cranial US was performed as soon as possible after admission to the neonatal unit, using a Kontron Sigma 1AC until 1993 and subsequently a Vingmed Diasonics in Lille and an ATL UM-4 in Utrecht with a 5-7.5-10 MHz transducer. Examinations were routinely performed two to three times during the first week of life and then once a week until discharge or more often as clinically indicated. Examinations were performed using the 7.5 MHz transducer in both units. The 10 MHz transducer was sometimes used when onset of cystic lesions was suspected. c-PVL was diagnosed as an area of increased echogenicity in the acute phase with a subsequent breakdown into echolucent areas within the following two to six weeks. c-PVL was classified according to the grading system of de Vries et al.5 Grade II leucomalacia was present if there were transient periventricular densities, evolving into small localised cysts (fig 1). Grade III leucomalacia was defined as areas of periventricular densities evolving into extensive periventricular cystic lesions (fig 2). All US pictures were independently reported by neonatologists (VP, CD, LdV) from both neonatal units, and agreement on classification was reached in all but two cases, which were subsequently excluded.
Coronal (A) and left parasagittal (B) views on day 4 in a preterm infant born at 29 weeks gestation, showing mild symmetrical periventricular densities. At 6 weeks of age, the coronal (C) and left parasagittal (D) views show a localised left sided occipital cyst (arrowhead) with associated mild ventriculomegaly. At 40 weeks postmenstrual age, in the right (E) and left (F) parasagittal views, the cyst is no longer seen and ventriculomegaly is only present on the left.
Coronal (A) and right parasagittal (B) views on day 24 in a preterm infant born at 28 weeks gestation, showing extensive cysts, more on the right than on the left side.
NEUROLOGICAL ASSESSMENT OF OUTCOME
Assessment of outcome was made on clinical examination at discharge using the standardised protocol of Amiel-Tison and Grenier17 in Lille and the Dubowitz assessment18 in Utrecht. Children were subsequently assessed at 6, 9, 12, 18, and 24 months corrected age, using items from Amiel-Tison and Stewart19 and Touwen.20Cerebral palsy was classified according to the criteria of Hagberget al.21
STATISTICAL ANALYSIS
The results of all comparisons are presented as relative risks (RR), odds ratios (OR), and 95% confidence intervals in parentheses.
Results
During the study period, c-PVL was diagnosed in 96 infants (2.8%). There was no significant difference in the prevalence between the centres (Lille 57/1824, 3.1%; Utrecht 39/1627, 2.4%; OR = 1.31 (0.85 to 2.03)). Grade II PVL was more often diagnosed in Lille (30/57) than in Utrecht (9/39) (OR = 3.89 (1.33 to 11.66)). Eighteen of the 96 infants were excluded from the study for the following reasons: seven had cysts on the first scan and were therefore considered to be of antenatal onset; nine were admitted after the first month of life with evidence of cysts on the first scan; no definite classification could be achieved in two cases. Of the remaining 78 infants, 39 had a grade II PVL and 39 had a grade III PVL. The two populations of grade II and grade III PVL were comparable with respect to gestational age and birth weight (table 1). The prevalence of both grade II and grade III PVL did not change with time.
Clinical and outcome data for infants with grade II and grade III periventricular leucomalacia
TIMING AND EVOLUTION OF THE CYSTIC LESIONS
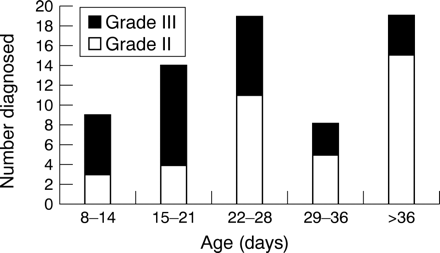
Figure 3 reports the age in days when the cysts in grade II and grade III PVL were first noted. Nine infants were not scanned on a regular enough basis to time the onset of the cysts.
The age in days when the cysts were first diagnosed is shown for children with grade II and grade III periventricular leucomalacia.
Grade II (n = 39)
Exact timing was not possible in only one infant. The echodensities were present at birth in all but one of the children. Cysts developed within the first 4 weeks of life in 18 of the 38 infants and in 20 infants at a later stage. The mean (SD) age when the cysts were diagnosed, following the development of echodensities, was 35 (16) days. Localisation of the cysts was in the parieto-occipital region in 8/39 infants. The frontal or parietal white matter was affected in 24 cases, and in seven infants the cysts were diagnosed in the occipital periventricular white matter. The cysts were unilateral in 21/39 infants, following the presence of bilateral echodensities. In the infants with unilateral cysts, the hyperechogenicity was usually asymmetrical, being more pronounced on the side where the cysts were subsequently seen. One infant was not seen at 40 weeks postmenstrual age (PMA) for a repeat US examination. In the other 38 infants, scanned at 40 weeks PMA, the cysts were no longer seen using US in 13, and mild ventriculomegaly was the only sequel present at this stage in nine. Ventriculomegaly was also present in four other infants in whom the cysts could still be seen.
Grade III (n = 39)
Exact timing was not possible in eight of the 39 infants. In all but seven cases, the echodensities were present at birth. Cysts developed within the first 4 weeks of life in 24 of the 31 infants and in seven at a later stage. The mean (SD) age when the cysts were first seen, following the appearance of echodensities, was 21 (10) days. The cysts were bilateral in all infants. Cysts were in the parieto-occipital region in 32/39 infants. In 20/32, the cysts extended into the frontal periventricular white matter. In the other seven infants, cysts were located in the frontoparietal (n = 6) or parietal area (n = 1). Twelve infants died before 40 weeks PMA. In two of the remaining 27 infants, cysts were no longer seen at 40 weeks PMA, using US, but ventriculomegaly was now noted to be present. Mild ventriculomegaly was also diagnosed in 17 infants in whom cysts could still be diagnosed.
NEURODEVELOPMENTAL OUTCOME
Grade II
One infant died during the first year of life, after discharge home. Twenty nine infants developed cerebral palsy, eight quadriplegia, 15 diplegia, and six hemiplegia. Twenty two of these infants have so far achieved independent walking at 18–30 months. All but one of the neonates with quadriplegia had parietal cysts, associated with frontal or occipital cysts in seven. The last one had occipital cysts only. Nine infants had transient tone abnormalities, but were considered to be normal at 2 years. Cysts were frontal in one of them, parietal in four, and occipital in four. Fourteen infants with unilateral cysts developed cerebral palsy, which was bilateral in nine of them: five hemiplegia, six diplegia, and three quadriplegia. Among the nine children who had no cysts around term but who had developed mild ventriculomegaly, seven developed cerebral palsy, and two were considered to be normal. One of these two had frontal cysts and the other one had small occipital cysts. Three of the other four infants, with a normal US scan at discharge—that is, no cysts and no ventriculomegaly—were normal at follow up, and one developed a hemiplegia.
Grade III
All but one of the 27 survivors with grade III PVL developed cerebral palsy. They were all associated with severe handicap and only three of them, with spastic diplegia, have so far achieved independent walking at 3–5 years. The other 23 had quadriplegia with severe restriction of spontaneous mobility. The last infant with extensive frontal cysts and localised occipital cysts did not develop cerebral palsy, but has a pronounced squint and learning disabilities, with a developmental quotient < 70 at 24 months corrected age.
Comparison of grade II and grade III PVL
Cysts were noted to develop more often after the first month of life (53%) in infants with grade II PVL than in those with grade III PVL (22%) (OR 3.81 (1.19 to 12.63)). Cysts were unilateral in 21 of the 39 infants with grade II PVL, whereas this was never seen in grade III PVL (OR indefinite; RR 3.17 (2.16 to 4.64)). At 40 weeks PMA, cysts were no longer seen using US in 13/38 infants with grade II PVL, with ventriculomegaly being the only visible sequel in nine cases. In grade III PVL, cysts were still present in 25 of the 27 surviving infants.
Nine infants with grade II PVL were free of motor sequelae at follow up compared with only one infant with grade III PVL (OR 8.07 (0.92 to 181.66)). Twenty two of 29 children with grade II PVL who developed cerebral palsy achieved independent walking compared with 3/26 with grade III PVL (OR 75 (11.4 to 662)).
Discussion
In this study, the US diagnosis and subsequent neurodevelopmental outcome of 39 infants with grade II PVL are reported and these data are compared with the data of a group of 39 infants with grade III PVL. In the cohorts described, the prevalence of c-PVL was low(2.8%) and comparable in Lille and Utrecht. The prevalence usually varies from one centre to the next and is classically related to diagnosing periventricular haemorrhagic venous infarction as PVL. Bass et al,22 being careful to differentiate between these two conditions, found a prevalence of 3.6% for PVL in a cohort of neonates with a birth weight below 1500 g. Perlmanet al 23 reported a prevalence of 2.3% c-PVL in a cohort of neonates of less than 1750 g, although late onset c-PVL could have been missed, as US was not obtained routinely after day 28. Zupan et al 24, using a US protocol comparable with the one used in this study, observed a higher incidence of 9.2% in a cohort of 802 infants born between 24 and 32 weeks. Baud et al 25 have shown considerable incidence differences among centres and suggested that the hospital recruitments and policies should be reported in more detail.
No distinction was made between grade II and grade III PVL in any of these recent studies. We were not able to explain the difference in the prevalence of grade II and grade III between our two units. Although different US machines were used, the two units had the same protocol, preferably using a 7.5 MHz transducer. We did not observe any variation with time in the prevalence of both grade II and grade III PVL, although appreciable changes in the care of low birthweight infants has been noted during the last 10 years. This supports the recent hypothesis that the pathophysiological cascade leading to PVL may be initiated before birth.26 ,27
Although according to our protocol the infants were only scanned once a week, we found a significant difference between the time when the cysts were first diagnosed. We could, however, not be more precise about the exact day when the cysts developed because of the limited number of US examinations. In infants with grade II PVL, cysts were noted to develop more often after the first month of life, in contrast with those with grade III PVL cases, who tended to develop cysts within the first 2–3 weeks of life. All seven infants with grade III PVL who developed cysts after day 28 had so called “late onset PVL”.28 ,29 The later development of cysts in grade II PVL was not due to late onset PVL, as echodensities were present on the first or second scan in all but one of the infants. Attention was previously paid to late onset PVL, often related to septicaemia or necrotising enterocolitis, and it was suggested that repeat US examinations should be performed after a second period of severe illness.28 ,29 Attention has so far not been drawn to the later occurrence of cysts in infants with grade II PVL, defined as PVL with smaller and more localised cysts compared with grade III PVL. In addition to the cysts being smaller, they are only present for a short time and it is not uncommon that they are no longer detectable by ultrasonography at 40 weeks PMA. Mild ventriculomegaly will then be the only sequel visible at this stage. These smaller cysts can therefore be easily missed, when infants are not scanned with a 7.5 MHz transducer and/or discharged within the first 2–3 weeks of life.
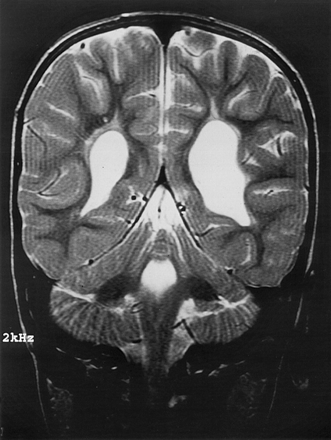
Grade II PVL was unilateral in more than half of the cases (54%) in spite of bilateral echodensities on US to start with. It was of interest that magnetic resonance imaging (MRI), performed in a small number of these cases, either in the neonatal period or at follow up,30 showed bilateral areas of abnormal signal intensity, suggestive of bilateral gliosis (fig 4).
Magnetic resonance image of the infant in fig 1, performed at 2 years of age. The T2 weighted SE sequence shows asymmetrical ventriculomegaly, more pronounced and irregular on the left, and bilateral gliosis (courtesy of Dr Bouman, Deventer).
The infants with grade III PVL always showed bilateral cysts. Most of the cystic lesions in grade III PVL (82%) were diagnosed in the parieto-occipital periventricular white matter compared with 20% of the infants with grade II PVL. In the initial report of Fawer and Calame,6 the focal or small lesions were all in the frontal periventricular white matter, while the large lesions were found in the frontoparietal or frontoparieto-occipital periventricular white matter. In other studies, small lesions were observed in the different periventricular areas.12 ,13 In fact, it seems that cysts in grade II PVL are more often diagnosed anteriorly but can also occur in the parietal or occipital periventricular white matter.
All but one of our infants with grade III PVL developed cerebral palsy and only three of them achieved independent walking. In comparison, cerebral palsy was diagnosed in 29/39 infants with grade II PVL but was less severe in this subgroup, as 22 achieved independent walking. Children with a normal outcome were more often present among those with grade II PVL and three of these nine infants did in fact have a normal US scan at 40 weeks PMA. In contrast with the infants with grade III PVL, no clear association could be found between the localisation of the cysts and the neurodevelopmental outcome in infants with grade II PVL.
The follow up data are in agreement with a recent review of the literature.16 In the pooled population described by Holling and Leviton,16 quadriplegia was associated with white matter lesions in frontal, parietal, or occipital lobes of both hemispheres. In contrast, only 35% of infants with “small” lesions and 61% with “medium” lesions developed cerebral palsy. Levene30 was the first to suggest that US scan performed around 40 weeks PMA has the best predictive value with regard to subsequent neurodevelopmental outcome. Recent studies4 ,31have focused on ventriculomegaly and suggested that it strongly indicates white matter disease. Paneth32 recently suggested inclusion of ventricular enlargement in the spectrum of white matter disease. It is quite possible that some of these infants with ventriculomegaly had grade II PVL, but that this diagnosis was not made because US examinations were performed during a limited number of weeks. In our cohort of infants with c-PVL, ventriculomegaly on its own was indeed a good predictor of cerebral palsy, as 29/30 infants with ventriculomegaly around term developed cerebral palsy. It is, however, important to bear in mind that ventriculomegaly can also be present at term in infants who had an intraventricular haemorrhage during the neonatal period. In these infants, ventriculomegaly may be due to mild posthaemorrhagic ventricular dilatation, which is unlikely to lead to an adverse neurological outcome.6 It is sometimes suggested that the enlarged ventricles, belonging to the spectrum of white matter disease, are more irregular in shape. The distinction between these two entities, which can also occur together, can in our experience only be confidently made when long term sequential US studies are available.
With the increasing use of MRI during the early neonatal period, it has become clear that cranial ultrasonography underdiagnoses the milder or diffuse lesions of white matter disease in particular.30 ,34 ,35 Sie et al 36 recently showed that conventional MRI was superior in identifying and quantifying cerebral white matter damage in very low birthweight infants. Some 71% of our population with unilateral cysts developed cerebral palsy with bilateral motor signs in 64%, compared with 76% and 55% in the pooled population described by Holling and Leviton.16 We were not able, however, to quantify the magnitude of hyperechogenicity, which could be more important than cysts in grade II PVL. Inder et al 37 recently showed earlier detection of the diffuse component of c-PVL with diffusion weighted MRI than with conventional brain imaging techniques. Diffusion weighted MRI, used at an early stage, could prove to be more reliable in predicting whether areas of increased echogenicity on US will evolve into cysts. At present, however, MRI cannot replace cranial ultrasonography as a routine tool in most neonatal units. We therefore strongly recommend that weekly US examinations are performed in all at risk infants until discharge, even when no obvious densities are apparent on previous US examinations.
References
Narrative Based Medicine, An Interdisciplinary Conference Research, Narrative, and Practice A two day conference—Monday 3rd and Tuesday 4th September 2001 Homerton College, Cambridge, UK
BMJ Publishing Group
For full details contact: BMA/BMJ Conference Unit, Tavistock Square, London, WC1H 9JP Tel: +44 (0)20 7383 6819; fax: +44 (0)20 7383 6663; email: clyders{at}bma.org.uk. www.quality.bmjpg.com