Article Text
Abstract
AIM To assess the change in stress response in preterm babies changed from patient triggered ventilation (PTV) to conventional mandatory ventilation (CMV) and vice versa; to determine outcome in relation to stress hormone concentrations.
METHODS A randomised controlled study was conducted in two district general hospital neonatal intensive care units. Thirty babies, treated initially with CMV, were randomly assigned to remain on CMV or to change to PTV. A second group of 29 babies, treated initially with PTV, were randomly assigned to remain on PTV or to change to CMV. The babies were less than 32 weeks of gestation, ventilated within 72 hours of birth, with clinical and radiological features compatible with respiratory distress syndrome (RDS). Stress hormone concentrations and clinical distress score were measured before and 20 minutes after allocation of mode of ventilation.
RESULTS Babies changed from CMV to PTV had significantly reduced adrenaline concentrations (median change −0.4 nmol/l) compared with those who remained on CMV. There was no increase in adrenaline in babies changed from PTV to CMV. There were no significant changes in noradrenaline concentrations or clinical distress score. Babies who died had significantly higher adrenaline and noradrenaline concentrations than those who survived.
CONCLUSION A change in mode of ventilation significantly reduces adrenaline concentrations. Raised catecholamine values are associated with a poor outcome.
- stress response
- trigger ventilation
- conventional ventilation
- hormones
Statistics from Altmetric.com
Patient triggered ventilation (PTV) is now feasible in preterm newborns as a result of improved airway sensors and significant reductions in lag time between patient effort and ventilator breath.1 2 Conventional mandatory ventilation (CMV) does not take account of the baby’s efforts, and asynchrony between baby and ventilator has been implicated as a factor in the development of pneumothorax3 and intraventricular haemorrhage.4 Sedatives and muscle paralysis have been used to reduce asynchrony but have not reduced the incidence of these adverse effects in clinical trials.5 6 Sedatives can also reduce stress hormone concentrations in CMV treated babies with no discernible effect on outcome.7 The aim of PTV is to provide synchrony between the baby and the ventilator, although improved synchrony has not been shown in practice. Nursing and medical staff have observed that babies seem to be more relaxed and comfortable on PTV than on CMV,1 but this has not been the subject of a controlled investigation.
There is no simple method of measuring a ventilated baby’s distress level. Clinical assessments that depend on facial expression are difficult because strapping and the endotracheal tube obscure the baby’s face. A clinical score has been validated in non-ventilated preterm babies which incorporates body movements as well as facial expression and cry (Neonatal Infant Pain Score or NIPS).8A recently developed score designed for use in ventilated babies has yet to be validated.9 Anand et al 10 11 have demonstrated the prognostic importance of endocrine stress hormone measurements which have since been widely used as short term indicators of stress in babies. Catecholamines have a short half life of 2 minutes12 and, therefore, provide an immediate measure of stress.
The aim of the present study was to determine whether a change in mode of ventilation in preterm babies over a relatively short time period produced a change in stress hormone concentrations. Because of the substantial variation in catecholamine concentrations within babies and the many different potential stressors, changes in concentrations were analysed over a relatively short time period during which this single change in management occurred. The hypothesis tested was that PTV is less stressful than CMV for babies with hyaline membrane disease, and that a change to this method of ventilation will result in a reduction in stress hormone concentrations compared with those infants in whom there is no change in mode of ventilation. Conversely, an increase in stress hormone concentrations would be expected to occur when ventilation is changed from PTV to CMV.
Methods
The study constituted a part of the Collaborative Randomised Trial of Trigger vs Conventional Ventilation. The entry criteria for this trial are: preterm babies <32 weeks of gestation, ventilated within 72 hours of birth, and clinical/radiological features compatible with respiratory distress syndrome (RDS). Babies in Exeter or Plymouth who were eligible were entered in the stress hormone study if parental consent had been obtained and they had a functioning arterial catheter to obtain blood samples non-invasively. Ethics committee permission was obtained in Exeter, Torbay (for babies transferred to Exeter from there), and Plymouth.
Babies were ventilated using the Draeger Babylog and SLE 2000 in Exeter and the SLE 2000 in Plymouth. Trigger ventilated babies had an initial peak inspiratory pressure (PIP) setting sufficient to produce chest wall movement and apparently synchronous comfortable breaths. The positive end expiratory pressure (PEEP) was set at 3–4 cm of water. The inspiratory time (I time) was 0.2 to 0.3 seconds and the backup rate 35 breaths/minute. The trigger sensitivity was adjusted to the optimal setting to produce the most effective triggering for that baby. Conventionally ventilated babies had an initial PIP to produce chest wall movement and a PEEP of 3–4 cm water. The I time and rate were 0.5 seconds and 40 breaths/minute, in Exeter and 0.35 seconds and 60 breaths/minute in Plymouth. Surfactant (ALEC) was given routinely as soon as possible after delivery to all babies ⩽ 28 weeks of gestation in Exeter and ⩽ 30 weeks of gestation in Plymouth. More mature babies were given ALEC if they required intubation for treatment of their hyaline membrane disease.
To create two different groups, babies were started on PTV (n=29) as their initial mode of ventilation from February to November 1994 and CMV (n=30) from December 1994 to August 1995. Babies transported from Torbay were ventilated using CMV for the duration of their transfer for both time periods. Babies were then randomised by the Collaborative Trial office in Plymouth either to stay on their current mode of ventilation or to change to the alternative. In the CMV group, 14 babies remained on CMV while 16 changed to PTV. Nineteen of those who started on PTV remained on PTV and 10 changed to CMV. Change in mode of ventilation involved turning a switch on the SLE 2000 or Draeger Babylog ventilators.
Blood samples were taken for catecholamine concentrations and blood gas analysis and a NIPS score was estimated immediately before and 20 minutes after the mode of ventilation was changed following randomisation. No nursing or medical procedures were performed on the babies during the 20 minute study period or for the 20 minutes before randomisation to minimise the effect of other factors on catecholamine. None of the babies was sedated or paralysed before or during the stress hormone study. Sedation with a continuous morphine infusion was subsequently prescribed if the baby was struggling and/or respiratory efforts were out of synchrony with the ventilator.
The blood for catecholamine concentrations was immediately centrifuged and the plasma stored at −70°C. The samples were transported in batches to Leeds for analysis using a radio enzymatic method.13
Statistical analysis was performed using Minitab–unpaired t test for comparing groups with normally distributed data (changes in stress hormone concentrations) and Mann Whitney U for non-parametric data (absolute stress hormone concentrations). Using data from a previous study,7 a power calculation predicted that 60 babies would need to be studied to have a 90% chance of showing a significant (p < 0.05) change in adrenaline of 0.4 nmol/l and noradrenaline of 2 nmol/l.
Results
Clinical data on the study babies are shown in table 1. The median (range) birthweight and gestation of the study group was 1035 g (440–2015) g and 28 weeks (23–31) weeks, respectively. There were no significant differences for any of these clinical variables among the four study subgroups.
Clinical data (median (range)) for four randomised study subgroups
Table 2 shows the catecholamine values in the CMV and PTV groups before randomisation. There was no significant difference between the groups.
Catecholamine values before randomisation (median ± IQ range)
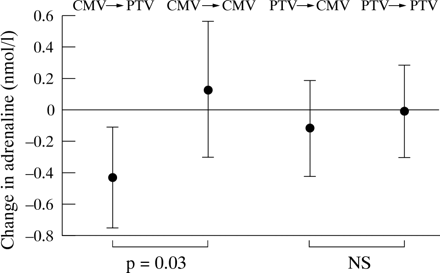
Figures 1 and 2 show the changes in catecholamine values between 0 and 20 minutes after allocation of ventilation in the four study groups. A change in mode of ventilation from CMV to PTV was accompanied by a significant reduction in adrenaline concentrations (mean change − 0.4 nmol/l) compared with those who stayed on CMV (median change + 0.1 nmol/l, p = 0.03). There was no corresponding increase in adrenaline in the group who were changed from PTV to CMV. There were no significant changes in noradrenaline concentrations in any of the study groups.
Change in adrenaline concentrations (mean(95% CI)) with change in mode of ventilation.
Change in noradrenaline concentrations (mean (95% CI)) with change in mode of ventilation.
Change in mode of ventilation was not accompanied by significant changes in arterial pO2 pCO2, FIO2or A:a ratio when the study groups were compared with their control groups (table 3). There were borderline significant reductions in A:a ratio [CMV→PTV group (p = 0.045)] and pCO2 [CMV→CMV group (p = 0.05)] within individual subgroups on paired testing.
Mean (± 95% CI) change in arterial pO2, pCO2, FIO2 and A:a ratio with change in mode of ventilation (negative figure denotes reduction)
The use of sedation with morphine was analysed according to the assigned mode of ventilation. Thirteen of 24 babies randomised to CMV received morphine; 16 of 35 babies assigned to PTV received morphine.
The average of the two catecholamine values was analysed in relation to death before discharge (table 4). The causes of death were severe hyaline membrane disease (n=11), septicaemia (n=3), chronic lung disease (n=2), hypoxic–ischaemic encephalopathy (n=1) and pulmonary atresia (n=1). Both adrenaline and noradrenaline concentrations were significantly higher in babies who subsequently died than in those who survived. The median difference between survivors and non-survivors was 0.8 nmol/l for adrenaline and 2.0 nmol/l for noradrenaline. The NIPS score was 0 in 53 of 59 babies at enrolment into the study. The median change was 0 in all four groups.
Catecholamine values (median ± IQ range) and outcome
Discussion
This study has shown a reduction in adrenaline concentrations over a short time period when mode of ventilation was changed from CMV to PTV. The reduction in adrenaline is equivalent to that observed after giving morphine to babies who remained on CMV in a previous study (median reduction = 0.4 nmol/l).7 This change is half the difference in adrenaline concentrations between survivors and non-survivors found in the present study and, therefore, may be significant. There was a reduction in A:a ratio in the CMV PTV group associated with a reduction in pO2, indicating worsening disease severity in this group. These changes are more likely to be associated with an increase in stress hormone concentrations.14 15 The reduction in adrenaline concentrations is, therefore, likely to reflect a genuine reduction in stress on changing from CMV to PTV, possibly due to a reduction in the number of asynchronous breaths. The failure of adrenaline concentrations to increase on changing from PTV to CMV may be due to the level of synchrony achieved on CMV during the short study period. During the 20 minutes on CMV these babies might have achieved good synchronisation with the ventilator breaths and, therefore, a change from PTV did not result in a significant increase in the number of asynchronous breaths. As baby breath/ventilator breath synchrony was not measured as a part of the study, this information is not available. There was no significant change in noradrenaline concentration over the short period of the study intervention, a finding consistent with other studies,10 suggesting that noradrenaline is more a measure of long term stress. This may be due to its method of production by secretion from stimulated sympathetic nerve endings and slow release into the blood stream. There is also a maturational effect in which preterm babies secrete relatively greater proportions of adrenaline than noradrenaline in the early days of life and are, therefore, more likely to respond to acute stress by an increase in adrenaline concentrations.16
There was no difference in baseline catecholamine concentrations between the CMV and PTV groups at enrolment. Babies were not randomly allocated to these groups and, furthermore, several babies treated with PTV were ventilated using CMV for their transfer from Torbay to Exeter. As so many factors influence catecholamine concentrations, a more sensitive measure of the effect of mode of ventilation is likely to be a short term change during which there are no other interventions, such as occurred in this study.
A further theoretical advantage of PTV is that it should reduce the need for sedatives by improving synchrony with the ventilator. Because of the very substantial variation in the pharmacokinetics of these drugs in premature babies, there may well be risks associated with their administration.17 18 The present study, however, showed no significant reduction in the use of sedatives in the trigger ventilated group.
This study has confirmed the findings of Barker and Rutter14 that raised catecholamine concentrations in ventilated preterm babies are associated with a poor outcome. Adults in septicaemic, traumatic, or haemorrhagic shock have similarly been found to have raised adrenaline and noradrenaline concentrations19 and those with persistently raised noradrenaline concentrations were more likely to die. It is unclear whether this is simply a measure of disease severity or whether the persistent stress hormone response is damaging in itself. There is evidence that a persistent stress response may be detrimental. A reduction in cardiac beta adrenoceptor density has been demonstrated in infants and children with persistently elevated catecholamine concentrations due to cardiac failure secondary to congenital heart disease.20 This resulted in a failure to respond to catecholamine treatment and would compromise the effectiveness of further increases in endogenous catecholamines in response to external stressors. Barker and Rutter’s study of preterm babies undergoing intensive care14 suggested that babies whose noradrenaline concentrations were reduced by treatment with diamorphine had a better outcome than those in whom it remained persistently high.
Previous studies have shown a reduction in stress hormone concentrations either by giving analgesia7 11 to babies and children undergoing intensive care or by providing soothing manoeuvres such as massage.21 One study has shown an improvement in survival in children who have been given high doses of opioids following cardiac surgery to suppress their endocrine stress response.11 The level of the stress response is likely to be much higher in these children than in the babies in the present study. It remains unresolved whether lesser manoeuvres to reduce stress in babies undergoing intensive care improves outcome.
Notably the NIPS score provided no useful information in assessing the level of stress in these babies. The score was validated in non-ventilated babies responding to an acute noxious insult. It is unreliable in assessing ventilated babies who are chronically stressed.
This study suggests a short term advantage of PTV over CMV in relation to stress hormone concentrations in ventilated preterm babies of less than 32 weeks of gestation. This advantage may not be seen in babies on CMV who are synchronising well with the ventilator. It seems likely than an even greater effect will occur in more mature babies. Of more importance than changes in stress hormone concentrations on different modes of ventilation is the outcome of these babies in relation to chronic lung disease and survival. This is the subject of an ongoing multicentre randomised trial.
Acknowledgments
This study was supported by a grant from the NHS South and West Research and Development Directorate.
We thank Dr Rod Taylor, Exeter and North Devon Research and Development Unit, for help with statistical analysis, and Dr Harvey Dean, University of Leeds for performing the essays.