Key Points
-
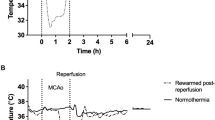
Small decreases in temperature can have remarkable effects on protecting the brain from ischaemic insults.
-
The mechanisms are multi-faceted, but span the spectrum of preserving cell metabolism, suppressing inflammation, increasing growth factor expression and favourably allowing regeneration and repair of the injured brain.
-
MicroRNAs and other temperature-sensitive RNAs may orchestrate the complex molecular events by selectively affecting gene expression to favour brain protection.
-
Therapeutic cooling can prevent complications of brain ischaemia such as oedema formation and brain haemorrhage.
-
Large prospective clinical studies have now demonstrated improvement in neurological outcome in some conditions of ischaemic brain injury.
-
Ongoing research continues to refine how therapeutic cooling may be delivered at the clinical level, including optimizing cooling conditions, studying related disease states and pharmacologic approaches.
Abstract
Cooling can reduce primary injury and prevent secondary injury to the brain after insults in certain clinical settings and in animal models of brain insult. The mechanisms that underlie the protective effects of cooling — also known as therapeutic hypothermia — are slowly beginning to be understood. Hypothermia influences multiple aspects of brain physiology in the acute, subacute and chronic stages of ischaemia. It affects pathways leading to excitotoxicity, apoptosis, inflammation and free radical production, as well as blood flow, metabolism and blood–brain barrier integrity. Hypothermia may also influence neurogenesis, gliogenesis and angiogenesis after injury. It is likely that no single factor can explain the neuroprotection provided by hypothermia, but understanding its myriad effects may shed light on important neuroprotective mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Busto, R. et al. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J. Cereb. Blood Flow Metab. 7, 729–738 (1987). This study was the first to show that relatively small decreases in temperature resulted in remarkable neuroprotection and led to a resurgence of interest in the field of therapeutic hypothermia.
Bernard, S. A. et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 346, 557–563 (2002).
The Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med. 346, 549–556 (2002). References 2 and 3 are prospective clinical studies that showed that mild hypothermia improved neurological outcomes in victims of cardiac arrest.
Gluckman, P. D. et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 365, 663–670 (2005). Aclinical trial of selective head cooling that showed improvement in neurological outcomes in some neonates with hypoxic ischaemic encephalopathy.
Shankaran, S. et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med. 353, 1574–1584 (2005). A clinical trial of whole body cooling that showed improvement in neurological outcomes in some neonates with hypoxic ischaemic encephalopathy.
Nozari, A. et al. Suspended animation can allow survival without brain damage after traumatic exsanguination cardiac arrest of 60 minutes in dogs. J. Trauma 57, 1266–1275 (2004).
Dietrich, W. D. & Bramlett, H. M. The evidence for hypothermia as a neuroprotectant in traumatic brain injury. Neurotherapeutics 7, 43–50 (2010).
Dietrich, W. D., Atkins, C. M. & Bramlett, H. M. Protection in animal models of brain and spinal cord injury with mild to moderate hypothermia. J. Neurotrauma 26, 301–312 (2009).
Colbourne, F., Sutherland, G. R. & Auer, R. N. An automated system for regulating brain temperature in awake and freely moving rodents. J. Neurosci. Methods 67, 185–190 (1996).
van der Worp, H. B., Sena, E. S., Donnan, G. A., Howells, D. W. & Macleod, M. R. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain 130, 3063–3074 (2007). This paper provided a systematic review and meta-analysis of the evidence for efficacy of hypothermia in animal models of ischaemic stroke based on more than 100 publications.
Krieger, D. W. & Yenari, M. A. Therapeutic hypothermia for acute ischemic stroke: what do laboratory studies teach us? Stroke 35, 1482–1489 (2004).
Yenari, M., Kitagawa, K., Lyden, P. & Perez-Pinzon, M. Metabolic downregulation: a key to successful neuroprotection? Stroke 39, 2910–2917 (2008). This paper reviews how therapeutic hypothermia and ischaemic preconditioning, which are both widely studied models of neuroprotection in the laboratory, have moved from basic science studies to clinical studies.
Colbourne, F., Li, H. & Buchan, A. M. Indefatigable CA1 sector neuroprotection with mild hypothermia induced 6 hours after severe forebrain ischemia in rats. J. Cereb. Blood Flow Metab. 19, 742–749 (1999). This study demonstrated remarkable and durable neuroprotection by therapeutic cooling in a global cerebral ischaemia model, even when cooling was started as late as 6 h from ischaemia onset.
Maier, C. M., Sun, G. H., Kunis, D., Yenari, M. A. & Steinberg, G. K. Delayed induction and long-term effects of mild hypothermia in a focal model of transient cerebral ischemia: neurological outcome and infarct size. J. Neurosurg. 94, 90–96 (2001).
Meloni, B. P., Mastaglia, F. L. & Knuckey, N. W. Therapeutic applications of hypothermia in cerebral ischaemia. Ther. Adv. Neurol. Disord. 1, 12–35 (2008).
Erecinska, M., Thoresen, M. & Silver, I. A. Effects of hypothermia on energy metabolism in mammalian central nervous system. J. Cereb. Blood Flow Metab. 23, 513–530 (2003).
Yenari, M., Wijman, C. & Steinberg, G. in Hypothermia in Neurocritical Care (eds Mayer, S. & Sessler, D.) 141–178 (Marcel Dekker, New York, 2004).
Zhao, H., Steinberg, G. K. & Sapolsky, R. M. General versus specific actions of mild-moderate hypothermia in attenuating cerebral ischemic damage. J. Cereb. Blood Flow Metab. 27, 1879–1894 (2007). This paper provides a comprehensive description of the protective mechanisms of hypothermia from laboratory data to facilitate clinical translation of therapeutic hypothermia.
Lee, J. M., Zipfel, G. J. & Choi, D. W. The changing landscape of ischaemic brain injury mechanisms. Nature 399, A7–A14 (1999).
Colbourne, F., Grooms, S. Y., Zukin, R. S., Buchan, A. M. & Bennett, M. V. Hypothermia rescues hippocampal CA1 neurons and attenuates down-regulation of the AMPA receptor GluR2 subunit after forebrain ischemia. Proc. Natl Acad. Sci. USA 100, 2906–2910 (2003).
Ginsberg, M. D., Sternau, L. L., Globus, M. Y., Dietrich, W. D. & Busto, R. Therapeutic modulation of brain temperature: relevance to ischemic brain injury. Cerebrovasc. Brain Metab. Rev. 4, 189–225 (1992).
Dietrich, W. D., Busto, R., Alonso, O., Globus, M. Y. & Ginsberg, M. D. Intraischemic but not postischemic brain hypothermia protects chronically following global forebrain ischemia in rats. J. Cereb. Blood Flow Metab. 13, 541–549 (1993).
Kamme, F., Campbell, K. & Wieloch, T. Biphasic expression of the fos and jun families of transcription factors following transient forebrain ischaemia in the rat. Effect of hypothermia. Eur. J. Neurosci. 7, 2007–2016 (1995).
Terao, Y. et al. Hypothermia enhances heat-shock protein 70 production in ischemic brains. Neuroreport 20, 745–749 (2009).
Cullen, K. E. & Sarge, K. D. Characterization of hypothermia-induced cellular stress response in mouse tissues. J. Biol. Chem. 272, 1742–1746 (1997).
Yenari, M. A. et al. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann. NY Acad. Sci. 1053, 74–83 (2005).
Kumar, K., Wu, X., Evans, A. T. & Marcoux, F. The effect of hypothermia on induction of heat shock protein (HSP)-72 in ischemic brain. Metab. Brain Dis. 10, 283–291 (1995).
Xu, L., Yenari, M. A., Steinberg, G. K. & Giffard, R. G. Mild hypothermia reduces apoptosis of mouse neurons in vitro early in the cascade. J. Cereb. Blood Flow Metab. 22, 21–28 (2002).
Vemuganti, R. The microRNAs and stroke: no need to be coded to be counted. Transl. Stroke Res. 1, 158–160 (2010).
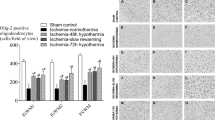
Truettner, J. S., Alonso, O. F., Bramlett, H. M. & Dietrich, W. D. Therapeutic hypothermia alters microRNA responses to traumatic brain injury in rats. J. Cereb. Blood Flow Metab. 31, 1897–1907 (2011). This paper demonstrated that several miRNAs are also extremely temperature sensitive (in addition to many well-known temperature-sensitive mRNAs).
Green, D. R. & Reed, J. C. Mitochondria and apoptosis. Science 281, 1309–1312 (1998).
Ashkenazi, A. & Dixit, V. M. Death receptors: signaling and modulation. Science 281, 1305–1308 (1998).
Liu, L. & Yenari, M. A. Therapeutic hypothermia: neuroprotective mechanisms. Front. Biosci. 12, 816–825 (2007).
Bright, R. et al. Protein kinase C δ mediates cerebral reperfusion injury in vivo. J. Neurosci. 24, 6880–6888 (2004).
Raval, A. P. et al. Protein kinase C δ cleavage initiates an aberrant signal transduction pathway after cardiac arrest and oxygen glucose deprivation. J. Cereb. Blood Flow Metab. 25, 730–741 (2005).
Lee, S. M., Zhao, H., Maier, C. M. & Steinberg, G. K. The protective effect of early hypothermia on PTEN phosphorylation correlates with free radical inhibition in rat stroke. J. Cereb. Blood Flow Metab. 29, 1589–1600 (2009).
Shimohata, T., Zhao, H. & Steinberg, G. K. ɛPKC may contribute to the protective effect of hypothermia in a rat focal cerebral ischemia model. Stroke 38, 375–380 (2007).
Martin-Villalba, A. et al. CD95 ligand (Fas-L/APO-1L) and tumor necrosis factor-related apoptosis-inducing ligand mediate ischemia-induced apoptosis in neurons. J. Neurosci. 19, 3809–3817 (1999).
Rosenbaum, D. M. et al. Fas (CD95/APO-1) plays a role in the pathophysiology of focal cerebral ischemia. J. Neurosci. Res. 61, 686–692 (2000).
Liu, L. et al. FasL shedding is reduced by hypothermia in experimental stroke. J. Neurochem. 106, 541–550 (2008).
Cunningham, L. A., Wetzel, M. & Rosenberg, G. A. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia 50, 329–339 (2005).
Powell, W. C., Fingleton, B., Wilson, C. L., Boothby, M. & Matrisian, L. M. The metalloproteinase matrilysin proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis. Curr. Biol. 9, 1441–1447 (1999).
Hamann, G. F. et al. Mild to moderate hypothermia prevents microvascular basal lamina antigen loss in experimental focal cerebral ischemia. Stroke 35, 764–769 (2004).
Lee, J. E., Yoon, Y. J., Moseley, M. E. & Yenari, M. A. Reduction in levels of matrix metalloproteinases and increased expression of tissue inhibitor of metalloproteinase-2 in response to mild hypothermia therapy in experimental stroke. J. Neurosurg. 103, 289–297 (2005).
Truettner, J. S., Alonso, O. F. & Dalton Dietrich, W. Influence of therapeutic hypothermia on matrix metalloproteinase activity after traumatic brain injury in rats. J. Cereb. Blood Flow Metab. 25, 1505–1516 (2005).
Wagner, S. et al. Topographically graded postischemic presence of metalloproteinases is inhibited by hypothermia. Brain Res. 984, 63–75 (2003).
Yenari, M. A. et al. Mild hypothermia attenuates cytochrome c release but does not alter Bcl-2 expression or caspase activation after experimental stroke. J. Cereb. Blood Flow Metab. 22, 29–38 (2002).
Susin, S. A. et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397, 441–446 (1999).
Zhao, H. et al. Conditions of protection by hypothermia and effects on apoptotic pathways in a rat model of permanent middle cerebral artery occlusion. J. Neurosurg. 107, 636–641 (2007).
Shi, G. D. et al. PTEN deletion prevents ischemic brain injury by activating the mTOR signaling pathway. Biochem. Biophys. Res. Commun. 404, 941–945 (2011).
Vosler, P. S., Logue, E. S., Repine, M. J. & Callaway, C. W. Delayed hypothermia preferentially increases expression of brain-derived neurotrophic factor exon III in rat hippocampus after asphyxial cardiac arrest. Brain Res. Mol. Brain Res. 135, 21–29 (2005).
D'Cruz, B. J. et al. Hypothermic reperfusion after cardiac arrest augments brain-derived neurotrophic factor activation. J. Cereb. Blood Flow Metab. 22, 843–851 (2002).
Schmidt, K. M., Repine, M. J., Hicks, S. D., DeFranco, D. B. & Callaway, C. W. Regional changes in glial cell line-derived neurotrophic factor after cardiac arrest and hypothermia in rats. Neurosci. Lett. 368, 135–139 (2004).
Boris-Moller, F., Kamme, F. & Wieloch, T. The effect of hypothermia on the expression of neurotrophin mRNA in the hippocampus following transient cerebral ischemia in the rat. Brain Res. Mol. Brain Res. 63, 163–173 (1998).
Hicks, S. D., Parmele, K. T., DeFranco, D. B., Klann, E. & Callaway, C. W. Hypothermia differentially increases extracellular signal-regulated kinase and stress-activated protein kinase/c-Jun terminal kinase activation in the hippocampus during reperfusion after asphyxial cardiac arrest. Neuroscience 98, 677–685 (2000).
D'Cruz, B. J., Logue, E. S., Falke, E., DeFranco, D. B. & Callaway, C. W. Hypothermia and ERK activation after cardiac arrest. Brain Res. 1064, 108–118 (2005).
Slikker, W., Desai, V. G., Duhart, H., Feuers, R. & Imam, S. Z. Hypothermia enhances bcl-2 expression and protects against oxidative stress-induced cell death in Chinese hamster ovary cells. Free Radic. Biol. Med. 31, 405–411 (2001).
Zhang, Z., Sobel, R. A., Cheng, D., Steinberg, G. K. & Yenari, M. A. Mild hypothermia increases Bcl-2 protein expression following global cerebral ischemia. Brain Res. Mol. Brain Res. 95, 75–85 (2001).
Zhao, H. et al. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J. Neurosci. 25, 9794–9806 (2005).
Wang, Q., Tang, X. N. & Yenari, M. A. The inflammatory response in stroke. J. Neuroimmunol. 184, 53–68 (2007).
Ceulemans, A. G. et al. The dual role of the neuroinflammatory response after ischemic stroke: modulatory effects of hypothermia. J. Neuroinflammation 7, 74 (2010).
Perrone, S. et al. Whole body hypothermia and oxidative stress in babies with hypoxic-ischemic brain injury. Pediatr. Neurol. 43, 236–240 (2010).
Han, H. S., Qiao, Y., Karabiyikoglu, M., Giffard, R. G. & Yenari, M. A. Influence of mild hypothermia on inducible nitric oxide synthase expression and reactive nitrogen production in experimental stroke and inflammation. J. Neurosci. 22, 3921–3928 (2002).
Deng, H., Han, H. S., Cheng, D., Sun, G. H. & Yenari, M. A. Mild hypothermia inhibits inflammation after experimental stroke and brain inflammation. Stroke 34, 2495–2501 (2003).
Meybohm, P. et al. Mild hypothermia alone or in combination with anesthetic post-conditioning reduces expression of inflammatory cytokines in the cerebral cortex of pigs after cardiopulmonary resuscitation. Crit. Care 14, R21 (2010).
Terao, Y. et al. Macrophage inflammatory protein-3α plays a key role in the inflammatory cascade in rat focal cerebral ischemia. Neurosci. Res. 64, 75–82 (2009).
Matsui, T. & Kakeda, T. IL-10 production is reduced by hypothermia but augmented by hyperthermia in rat microglia. J. Neurotrauma 25, 709–715 (2008).
Truettner, J. S., Suzuki, T. & Dietrich, W. D. The effect of therapeutic hypothermia on the expression of inflammatory response genes following moderate traumatic brain injury in the rat. Brain Res. Mol. Brain Res. 138, 124–134 (2005).
Yenari, M. A. & Han, H. S. Influence of hypothermia on post-ischemic inflammation: role of nuclear factor kappa B (NFκB). Neurochem. Int. 49, 164–169 (2006).
Han, H. S., Karabiyikoglu, M., Kelly, S., Sobel, R. A. & Yenari, M. A. Mild hypothermia inhibits nuclear factor-κB translocation in experimental stroke. J. Cereb. Blood Flow Metab. 23, 589–598 (2003).
Webster, C. M. et al. Inflammation and NFκB activation is decreased by hypothermia following global cerebral ischemia. Neurobiol. Dis. 33, 301–312 (2009).
Mattson, M. P., Culmsee, C., Yu, Z. & Camandola, S. Roles of nuclear factor κB in neuronal survival and plasticity. J. Neurochem. 74, 443–456 (2000).
Schmitt, K. R. et al. Hypothermia suppresses inflammation via ERK signaling pathway in stimulated microglial cells. J. Neuroimmunol. 189, 7–16 (2007).
Choi, J. S. et al. Mild hypothermia attenuates intercellular adhesion molecule-1 induction via activation of extracellular signal-regulated kinase-1/2 in a focal cerebral ischemia model. Stroke Res. Treat. 2011, 846716 (2011).
Dietrich, W. D., Busto, R., Halley, M. & Valdes, I. The importance of brain temperature in alterations of the blood-brain barrier following cerebral ischemia. J. Neuropathol. Exp. Neurol. 49, 486–497 (1990).
Kawanishi, M. et al. Effect of delayed mild brain hypothermia on edema formation after intracerebral hemorrhage in rats. J. Stroke Cerebrovasc. Dis. 17, 187–195 (2008).
MacLellan, C. L., Davies, L. M., Fingas, M. S. & Colbourne, F. The influence of hypothermia on outcome after intracerebral hemorrhage in rats. Stroke 37, 1266–1270 (2006).
Oda, Y., Gao, G., Wei, E. P. & Povlishock, J. T. Combinational therapy using hypothermia and the immunophilin ligand FK506 to target altered pial arteriolar reactivity, axonal damage, and blood-brain barrier dysfunction after traumatic brain injury in rat. J. Cereb. Blood Flow Metab. 31, 1143–1154 (2011).
Preston, E. & Webster, J. A two-hour window for hypothermic modulation of early events that impact delayed opening of the rat blood-brain barrier after ischemia. Acta Neuropathol. 108, 406–412 (2004).
Nagel, S. et al. Minocycline and hypothermia for reperfusion injury after focal cerebral ischemia in the rat: effects on BBB breakdown and MMP expression in the acute and subacute phase. Brain Res. 1188, 198–206 (2008).
Lee, K. M., Jang, J. H., Park, J. S., Kim, D. S. & Han, H. S. Effect of mild hypothermia on blood brain barrier disruption induced by oleic acid in rats. Genes Genomics 31, 89–98 (2009).
Baumann, E., Preston, E., Slinn, J. & Stanimirovic, D. Post-ischemic hypothermia attenuates loss of the vascular basement membrane proteins, agrin and SPARC, and the blood-brain barrier disruption after global cerebral ischemia. Brain Res. 1269, 185–197 (2009).
Duz, B., Oztas, E., Erginay, T., Erdogan, E. & Gonul, E. The effect of moderate hypothermia in acute ischemic stroke on pericyte migration: an ultrastructural study. Cryobiology 55, 279–284 (2007).
Manley, G. T. et al. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nature Med. 6, 159–163 (2000).
Dai, D. W. et al. Effect of local mild hypothermia on expression of aquaporin-4 following intracerebral hemorrhage in rats. Zhonghua Yi Xue Za Zhi 86, 906–910 (2006) (in Chinese).
Xiao, F. et al. Cerebral cortical aquaporin-4 expression in brain edema following cardiac arrest in rats. Acad. Emerg. Med. 11, 1001–1007 (2004).
Jin, J. S. et al. Effect of therapeutic moderate hypothermia on multi-drug resistance protein 1-mediated transepithelial transport of drugs. Neurol. Med. Chir. 46, 321–327 (2006).
Fingas, M., Penner, M., Silasi, G. & Colbourne, F. Treatment of intracerebral hemorrhage in rats with 12 h, 3 days and 6 days of selective brain hypothermia. Exp. Neurol. 219, 156–162 (2009). This is a comprehensive study of therapeutic hypothermia in a brain haemorrhage model.
Kollmar, R. et al. Hypothermia reduces perihemorrhagic edema after intracerebral hemorrhage. Stroke 41, 1684–1689 (2010).
Martini, W. Z. Coagulopathy by hypothermia and acidosis: mechanisms of thrombin generation and fibrinogen availability. J. Trauma 67, 202–209 (2009).
Martini, W. Z. The effects of hypothermia on fibrinogen metabolism and coagulation function in swine. Metabolism 56, 214–221 (2007).
Kernie, S. G. & Parent, J. M. Forebrain neurogenesis after focal ischemic and traumatic brain injury. Neurobiol. Dis. 37, 267–274 (2010).
Font, M. A., Arboix, A. & Krupinski, J. Angiogenesis, neurogenesis and neuroplasticity in ischemic stroke. Curr. Cardiol. Rev. 6, 238–244 (2010).
Shruster, A., Melamed, E. & Offen, D. Neurogenesis in the aged and neurodegenerative brain. Apoptosis 15, 1415–1421 (2010).
Jinno, S. Decline in adult neurogenesis during aging follows a topographic pattern in the mouse hippocampus. J. Comp. Neurol. 519, 451–466 (2011).
Kanagawa, T. et al. A decrease of cell proliferation by hypothermia in the hippocampus of the neonatal rat. Brain Res. 1111, 36–40 (2006).
Xiong, M. et al. Post-ischemic hypothermia promotes generation of neural cells and reduces apoptosis by Bcl-2 in the striatum of neonatal rat brain. Neurochem. Int. 58, 625–633 (2011).
Saito, K. et al. Moderate low temperature preserves the stemness of neural stem cells and suppresses apoptosis of the cells via activation of the cold-inducible RNA binding protein. Brain Res. 1358, 20–29 (2010).
Silasi, G. & Colbourne, F. Therapeutic hypothermia influences cell genesis and survival in the rat hippocampus following global ischemia. J. Cereb. Blood Flow Metab. 31, 1725–1735 (2011). This paper showed that prolonged hypothermia positively interacts with post-ischaemic repair processes, such as neurogenesis, resulting in improved functional outcome.
Lasarzik, I. et al. Mild hypothermia has no long-term impact on postischemic neurogenesis in rats. Anesth. Analg. 109, 1632–1639 (2009).
Lotocki, G. et al. Oligodendrocyte vulnerability following traumatic brain injury in rats. Neurosci. Lett. 499, 143–148 (2011).
Lotocki, G. et al. Oligodendrocyte vulnerability following traumatic brain injury in rats: effect of moderate hypothermia. Therapeutic Hypothermia and Temperature Management 1, 43–51 (2011).
Imada, S. et al. Hypothermia-induced increase of oligodendrocyte precursor cells: possible involvement of plasmalemmal voltage-dependent anion channel 1. J. Neurosci. Res. 88, 3457–3466 (2010).
Bennet, L. et al. The effect of cerebral hypothermia on white and grey matter injury induced by severe hypoxia in preterm fetal sheep. J. Physiol. 578, 491–506 (2007).
Matijasevic, Z., Snyder, J. E. & Ludlum, D. B. Hypothermia causes a reversible, p53-mediated cell cycle arrest in cultured fibroblasts. Oncol. Res. 10, 605–610 (1998).
Gopurappilly, R. et al. Stem cells in stroke repair: current success & future prospects. CNS Neurol. Disord. Drug Targets 10, 741–756 (2011).
Li, L. et al. Focal cerebral ischemia induces a multilineage cytogenic response from adult subventricular zone that is predominantly gliogenic. Glia 58, 1610–1619 (2010).
Hawthorne, A. L. et al. The unusual response of serotonergic neurons after CNS injury: lack of axonal dieback and enhanced sprouting within the inhibitory environment of the glial scar. J. Neurosci. 31, 5605–5616 (2011).
Trendelenburg, G. & Dirnagl, U. Neuroprotective role of astrocytes in cerebral ischemia: focus on ischemic preconditioning. Glia 50, 307–320 (2005).
Xie, Y. C., Li, C. Y., Li, T., Nie, D. Y. & Ye, F. Effect of mild hypothermia on angiogenesis in rats with focal cerebral ischemia. Neurosci. Lett. 422, 87–90 (2007).
Kao, C. H., Chio, C. C., Lin, M. T. & Yeh, C. H. Body cooling ameliorating spinal cord injury may be neurogenesis-, anti-inflammation- and angiogenesis-associated in rats. J. Trauma 70, 885–893 (2011).
Kuo, J. R. et al. Brain cooling-stimulated angiogenesis and neurogenesis attenuated traumatic brain injury in rats. J. Trauma 69, 1467–1472 (2010).
Navarro-Sobrino, M. et al. A large screening of angiogenesis biomarkers and their association with neurological outcome after ischemic stroke. Atherosclerosis 216, 205–211 (2011).
Manoonkitiwongsa, P. S., Schultz, R. L., McCreery, D. B., Whitter, E. F. & Lyden, P. D. Neuroprotection of ischemic brain by vascular endothelial growth factor is critically dependent on proper dosage and may be compromised by angiogenesis. J. Cereb. Blood Flow Metab. 24, 693–702 (2004).
Schmitt, K. R. et al. Hypothermia-induced neurite outgrowth is mediated by tumor necrosis factor-α. Brain Pathol. 20, 771–779 (2010).
Schmitt, K. R. et al. S100B modulates IL-6 release and cytotoxicity from hypothermic brain cells and inhibits hypothermia-induced axonal outgrowth. Neurosci. Res. 59, 168–173 (2007).
Feng, J. F. et al. Effect of therapeutic mild hypothermia on the genomics of the hippocampus after moderate traumatic brain injury in rats. Neurosurgery 67, 730–742 (2010).
Sessler, D. I. Complications and treatment of mild hypothermia. Anesthesiology 95, 531–543 (2001).
Frerichs, K. U. & Hallenbeck, J. M. Hibernation in ground squirrels induces state and species-specific tolerance to hypoxia and aglycemia: an in vitro study in hippocampal slices. J. Cereb. Blood Flow Metab. 18, 168–175 (1998).
Dave, K. R. et al. Protein kinase C epsilon activation delays neuronal depolarization during cardiac arrest in the euthermic arctic ground squirrel. J. Neurochem. 110, 1170–1179 (2009).
Drew, K. L. et al. Hypoxia tolerance in mammalian heterotherms. J. Exp. Biol. 207, 3155–3162 (2004).
Toien, O. et al. Hibernation in black bears: independence of metabolic suppression from body temperature. Science 331, 906–909 (2011).
Han, H. S. & Yenari, M. A. Effect of gene expression by therapeutic hypothermia in cerebral ischemia. Future Neurol. 2, 435–440 (2007). This review covers the spectrum of gene expression changes resulting from hypothermia, including temperature-sensitive RNAs.
Al-Fageeh, M. B. & Smales, C. M. Cold-inducible RNA binding protein (CIRP) expression is modulated by alternative mRNAs. RNA 15, 1164–1176 (2009).
Liu, A., Zhang, Z., Li, A. & Xue, J. Effects of hypothermia and cerebral ischemia on cold-inducible RNA-binding protein mRNA expression in rat brain. Brain Res. 1347, 104–110 (2010).
Sakurai, T. et al. Cirp protects against tumor necrosis factor-α-induced apoptosis via activation of extracellular signal-regulated kinase. Biochim. Biophys. Acta 1763, 290–295 (2006).
Acknowledgements
This work was supported by grants from the US National Institutes of Health (NS40516 to M.Y.), the Veteran's Merit Award (M.Y.), the Korea Healthcare technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A100870 to H.S.H.) and the Industrial Strategic technology development program (10035197 to H.S.H.), which is funded by the Ministry of Knowledge Economy (MKE), Korea. Grants to M.Y. were administered by the Northern California Institute for Research and Education, and supported by resources of the Veterans Affairs Medical Center, San Francisco, California.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Apoptosis
-
Innate, programmed cell death that is energy-dependent and leads to nuclear and cytoplasmic compaction with characteristic blebbing of the nucleus. It occurs during development but also in disease states.
- Necrosis
-
Acute, uncontrolled cell death that leads to cell lysis.
- Reperfusion
-
The period of resumed blood flow to the tissue after arterial occlusion.
- Hyperaemia
-
Higher than normal blood flow.
- Torpor
-
A prolonged state of energy conservation that allows heterothermic animals to tolerate limitations in resource availability that are encountered in extreme environments.
- Nestin
-
An intermediate filament protein used as a marker for CNS stem cells.
- Coagulopathies
-
Abnormal conditions of blood clotting or blood clot lysis.
- Autophagy
-
The breakdown of a cell's own components by the lysosome.
- Anoikis
-
A form of programmed cell death that is activated when cells detach from the extracellular matrix
Rights and permissions
About this article
Cite this article
Yenari, M., Han, H. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci 13, 267–278 (2012). https://doi.org/10.1038/nrn3174
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3174
This article is cited by
-
Therapeutic hypothermia modulates the neurogenic response of the newborn piglet subventricular zone after hypoxia-ischemia
Pediatric Research (2024)
-
Covalently Binding Adenosine A3 Receptor Agonist ICBM Irreversibly Reduces Voltage-Gated Ca2+ Currents in Dorsal Root Ganglion Neurons
Purinergic Signalling (2024)
-
The combination treatment of hypothermia and intranasal insulin ameliorates the structural and functional changes in a rat model of traumatic brain injury
Brain Structure and Function (2024)
-
RBM3 Promotes Anti-inflammatory Responses in Microglia and Serves as a Neuroprotective Target of Ischemic Stroke
Molecular Neurobiology (2024)
-
Optimizing intra-arterial hypothermia scheme for acute ischemic stroke in an MCAO/R rat model
Scientific Reports (2023)