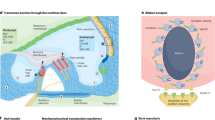
Abstract
Little is known of the molecular basis of normal auditory function. In contrast to the visual or olfactory senses, in which reasonable amounts of sensory tissue can be gathered, the auditory system has proven difficult to access through biochemical routes, mainly because such small amounts of tissue are available for analysis. Key molecules, such as the transduction channel, may be present in only a few tens of copies per sensory hair cell, compounding the difficulty. Moreover, fundamental differences in the mechanism of stimulation and, most importantly, the speed of response of audition compared with other senses means that we have no well-understood models to provide good candidate molecules for investigation. For these reasons, a genetic approach is useful for identifying the key components of auditory transduction, as it makes no assumptions about the nature or expression level of molecules essential for hearing. We review here some of the major advances in our understanding of auditory function resulting from the recent rapid progress in identification of genes involved in deafness.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Bob Crimi

Bob Crimi


Bob Crimi
Similar content being viewed by others
References
Petit, C. Genes responsible for human hereditary deafness: symphony of a thousand. Nature Genet. 14, 385–391 (1996).
Davis, A.C. Hearing in Adults (Whurr, London, 1995).
Steel, K.P., Erven, A. & Kiernan, A.E. Mice as models for human hereditary deafness. in Genetics and Auditory Disorders (eds. Keats, B., Fay, R. & Popper, A.N.) (Springer, New York, in press).
Gibson, F. et al. A type VII myosin encoded by the mouse deafness gene shaker-1. Nature 374, 62–64 (1995).
Probst, F.J. et al. Correction of deafness in shaker-2 mice by an unconventional myosin in a BAC transgene. Science 280, 1444–1447 (1998).
Minowa, O. et al. Altered cochlear fibrocytes in a mouse model of DFN3 nonsyndromic deafness. Science 285, 1408–1411 (1999).
Phippard, D., Lu, L., Lee, D., Saunders, J.C. & Crenshaw, E.B. III Targeted mutagenesis of the POU-domain gene Brn4/Pou3f4 causes developmental defects in the inner ear. J. Neurosci. 19, 5980–5989 (1999).
Erkman, L., McEvilly, R.J., Luo, L. & Ryan, A.K. Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature 381, 603–606 (1996).
McGuirt, W.T. et al. Mutations in COL11A2 cause non-syndromic hearing loss (DFNA13). Nature Genet. 23, 413–419 (1999).
Legan, P.K. et al. A targeted deletion in α-tectorin reveals that the tectorial membrane is required for the gain and timing of cochlear feedback. Neuron 28, 273–285 (2000).
Di Palma, F. et al. Mutations in Cdh23, encoding a new type of cadherin, cause stereocilia disorganization in waltzer, the mouse model for Usher syndrome type 1D. Nature Genet. 27, 103–107 (2001).
Everett, L.A. et al. Targeted disruption of mouse Pds provides insight about the inner-ear defects encountered in Pendred syndrome. Hum. Mol. Genet. 10, 153–161 (2001).
Corey, D.P. & Hudspeth, A.J. Kinetics of the receptor current in bullfrog saccular hair cells. J. Neurosci. 3, 962–976 (1983).
Howard, J. & Hudspeth, A.J. Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog's saccular hair cell. Neuron 1, 189–199 (1988).
Holt, J.R. & Corey, D.P. Two mechanisms for transducer adaptation in vertebrate hair cells. Proc. Natl. Acad. Sci. USA 97, 11730–11735 (2000).
Avraham, K.B. et al. The mouse Snell's waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner hair cells. Nature Genet. 11, 369–375 (1995).
Self, T. et al. Shaker-1 mutations reveal roles for myosin VIIA in both development and function of cochlear hair cells. Development 125, 557–566 (1998).
Self, T. et al. Role of myosin VI in the development of cochlear hair cells. Dev. Biol. 214, 331–341 (1999).
Weil, D. et al. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature 374, 60–61 (1995).
Weil, D. et al. The autosomal recessive isolated deafness, DFNB2, and the Usher 1B syndrome are allelic defects of the myosin-VIIA gene. Nature Genet. 16, 191–193 (1997).
Liu, X.-Z. et al. Mutations in the myosin VIIA gene causing non-syndromic recessive deafness. Nature Genet. 16, 188–190 (1997).
Wang, A. et al. Association of unconventional myosin MYO15 mutations with human nonsyndromic deafness DFNB3. Science 280, 1447–1451 (1998).
Wells, A.L. et al. Myosin VI is an actin-based motor that moves backwards. Nature 401, 505–508 (1999).
Richardson, G.P. et al. A missense mutation in myosin VIIA prevents aminoglycoside accumulation in early postnatal cochlear hair cells. Ann. NY Acad. Sci. 884, 110–124 (1999).
Küssel-Andermann, P. et al. Vezatin, a novel transmembrane protein, bridges myosin VIIA to the cadherin-catenins complex. EMBO J. 19, 6020–6029 (2000).
Verpy, E. et al. A defect in harmonin, a PDZ domain-containing protein expressed in the inner ear sensory hair cells, underlies Usher syndrome type 1C. Nature Genet. 26, 51–55 (2000).
Bitner-Glindzicz, M. et al. A recessive contiguous gene delection causing infantile hyperinsulinism, enteropathy and deafness identifies the Usher type 1C gene. Nature Genet. 26, 56–60 (2000).
Alagramam, K.N. et al. The mouse Ames waltzer hearing-loss mutant is caused by mutation of Pcdh15, a novel protocadherin gene. Nature Genet. 27, 99–102 (2001).
Zheng, L. et al. The deaf jerker mouse has a mutation in the gene encoding the espin actin-bundling proteins of hair cell stereocilia and lacks espins. Cell 102, 377–385 (2000).
Ohmori, H. Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J. Physiol. 359, 189–217 (1985).
Ricci, A.J. & Fettiplace, R. Calcium permeation of the turtle hair cell mechanotransducer channel and its relation to the composition of endolymph. J. Physiol. 506, 159–173 (1998).
Yamoah, E.N. et al. Plasma membrane Ca2+-ATPase extrudes Ca2+ from hair cell stereocilia. J. Neurosci. 18, 610–624 (1998).
Street, V.A., McKee-Johnson, J.W., Fonseca, R.C., Tempel, B.L. & Noben-Trauth, K. Mutations in a plasma membrane Ca2+-ATPase gene cause deafness in deafwaddler mice. Nature Genet. 19, 390–394 (1998).
Kozel, P.J. et al. Balance and hearing deficits in mice with a null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2. J. Biol. Chem. 273, 18693–18696 (1998).
Yasunaga, S. et al. A mutation in OTOF, encoding otoferlin, a FER-1-like protein, causes DFNB9, a non-syndromic form of deafness. Nature Genet. 21, 363–369 (1999).
Platzer, J. et al. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell 102, 89–97 (2000).
Géléoc, G.S.G., Casalotti, S.O., Forge, A. & Ashmore, J.F. A sugar transporter as a candidate for the outer hair cell motor. Nature Neurosci. 2, 713–719 (1999).
Zheng, J. et al. Prestin is the motor protein of cochlear outer hair cells. Nature 405, 149–155 (2000).
Belyantseva, I.A., Adler, H.J., Curi, R., Frolenkov, G.I. & Kachar, B. Expression and localisation of prestin and the sugar transporter GLUT-5 during development of electromotility in cochlear outer hair cells. J. Neurosci. 20, RC116 (2000).
Walker, R.G., Willingham, A.T. & Zuker, C.S. A Drosophila mechanosensory transduction channel. Science 287, 2229–2234 (2000).
Simmler, M.C. et al. Targeted disruption of Otog results in deafness and severe imbalance. Nature Genet. 24, 139–143 (2000).
Verhoeven, K. et al. Mutations in the human α-tectorin gene cause autosomal dominant non-syndromic hearing impairment. Nature Genet. 19, 60–62 (1998).
Steel, K.P., Barkway, C. & Bock, G.R. Strial dysfunction in mice with cochleo-saccular abnormalities. Hear. Res. 27, 11–26 (1987).
Johnstone, B.M., Patuzzi, R., Syka, J. & Sykova, E. Stimulus-related potassium changes in the organ of Corti of guinea-pig. J. Physiol. 408, 77–92 (1989).
Konishi, T., Harick, P.E. & Walsh, P.J. Ion transport in guinea pig cochlea. I. Potassium and sodium transport. Acta Otolaryngol. 86, 22–34 (1978).
Wada, J., Kambayashi, J., Marcus, D.C. & Thalmann, R. Vascular perfusion of the cochlea: effect of potassium-free and rubidium-substituted media. Arch. Otorhinolaryngol. 225, 79–81 (1979).
Kikuchi, T., Kimura, R.S., Paul, D.L. & Adams, J.C. Gap junctions in the rat cochlea: immunohistochemical and ultrastructural analysis. Anat. Embryol. 191, 101–118 (1995).
Spicer, S.S. & Schulte, B.A. Evidence for a medial K+ recycling pathway from inner hair cells. Hear. Res. 118, 1–12 (1998).
Schulte, B.A. & Steel, K.P. Expression of α and β subunit isoforms of Na,K-ATPase in the mouse inner ear and changes with mutations at the Wv or Sld loci. Hear. Res. 78, 65–76 (1994).
Kubisch, C. et al. KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell 96, 437–446 (1999).
Marcotti, W. & Kros, C.J. Developmental expression of the potassium current IK,n contributes to maturation of mouse outer hair cells. J. Physiol. 520, 653–660 (1999).
Housley, G.D. & Ashmore, J.F. Ionic currents of outer hair cells isolated from the guinea pig cochlea. J. Physiol. 448, 73–98 (1992).
Jentsch, T.J. Neuronal KCNQ potassium channels: physiology and role in disease. Nature Rev. Neurosci. 1, 21–30 (2000).
Kharkovets, T. et al. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc. Natl. Acad. Sci. USA 97, 4333–4338 (2000).
Beisel, K.W., Nelson, N.C., Delimont, D.C. & Fritzsch, B. Longitudinal gradients of KCNQ4 expression in spiral ganglion and cochlear hair cells correlate with progressive hearing loss in DFNA2. Brain Res. Mol. Brain Res. 82, 137–149 (2000).
Kelsell, D.P. et al. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 387, 80–83 (1997).
Xia, J. et al. Mutations in the gene encoding gap junction protein β-3 associated with autosomal dominant hearing impairment. Nature Genet. 21, 363–369 (1998).
Grifa, A. et al. Mutations in GJB6 cause nonsyndromic autosomal dominant deafness at DFNA3 locus. Nature Genet. 23, 16–18 (1999).
Wangemann, P. Comparison of ion transport mechanisms between vestibular dark cells and strial marginal cells. Hear. Res. 90, 149–157 (1995).
Dixon, M.J. et al. Mutation of the Na-K-Cl co-transporter gene Slc12a2 results in deafness in mice. Hum. Mol. Genet. 8, 1579–1584 (1999).
Delpire, E., Lu, J., England, R. & Thorne, T. Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nature Genet. 22, 192–195 (1999).
Flagella, M. et al. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J. Biol. Chem. 274, 26946–26955 (1999).
Vetter, D.E. et al. Inner ear defects induced by null mutation of the isk gene. Neuron 17, 1251–1264 (1996).
Tyson, J. et al. IsK and KvLQT1: Mutation in either of the two subunits of the slow component of the delayed rectifier potassium channel can cause Jervell and Lange-Nielsen syndrome. Hum. Mol. Genet. 6, 2179–2185 (1997).
Letts, V.A. et al. A new spontaneous mouse mutation in the Kcne1 gene. Mamm. Genome 11, 831–835 (2000).
Karet, F.E. et al. Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nature Genet. 21, 84–90 (1999).
Everett, L.A. et al. Pendred syndrome is caused by mutations in a putative sulphate transporter (PDS). Nature Genet. 17, 411–422 (1997).
Li, X.C. et al. A mutation in PDS causes non-syndromic recessive deafness. Nature Genet. 18, 215–217 (1998).
Everett, L.A., Morsli, H., Wu, D.K. & Green, E.D. Expression pattern of the mouse ortholog of the Pendred's syndrome gene ( Pds ) suggests a key role for pendrin in the inner ear. Proc. Natl. Acad. Sci. USA 96, 9727–9732 (1999).
Acknowledgements
Supported by the MRC, Defeating Deafness and EC contract QLG2-CT-1999-00988.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steel, K., Kros, C. A genetic approach to understanding auditory function. Nat Genet 27, 143–149 (2001). https://doi.org/10.1038/84758
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/84758
This article is cited by
-
Knowledge of genetics of hearing and genetic counseling among practicing audiologists
Egyptian Journal of Medical Human Genetics (2023)
-
Comparison of Otoacoustic Emission (OAE) and Brainstem Evoked Response Audiometry (BERA) in High Risk Infants and Children under 5 Years of Age for Hearing Assessment in Western India: A Modification in Screening Protocol
Indian Journal of Otolaryngology and Head & Neck Surgery (2022)
-
Universal newborn hearing screening: methods and results, obstacles, and benefits
Pediatric Research (2017)
-
Distinct Expression Patterns Of Causative Genes Responsible For Hereditary Progressive Hearing Loss In Non-Human Primate Cochlea
Scientific Reports (2016)
-
Analysis of association among clinical features and shorter leukocyte telomere length in mitochondrial diabetes with m.3243A>G mitochondrial DNA mutation
BMC Medical Genetics (2015)