Abstract.
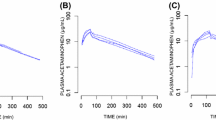
Background: Pharmacodynamic models of acetaminophen analgesia in children have not explored the efficacy of single oral doses greater than 40 mg/kg. Methods: Children aged 9.0±3.0 years (±SD) and weight 37.9±16.6 kg undergoing outpatient tonsillectomy were randomised to receive acetaminophen elixir 40 mg/kg (n=12), high dose acetaminophen elixir 100 mg/kg (n=20) or placebo (n=30) 0.5–1 h preoperatively. No other analgesics were given. Individual acetaminophen serum concentrations and pain scores [visual analogue scale (VAS) 0–10] were measured over a 4–8 h postoperative period. These data were pooled with data from a previous study investigating acetaminophen pharmacodynamics (n=120) and analysed using a non-linear mixed effect model. Placebo effects and drug effects were modelled using effect-site concentration models. Results: A one-compartment model with first-order input, lag time and first-order elimination was used to describe the population pharmacokinetics of acetaminophen. Pharmacokinetic parameter estimates were similar to those previously described. Pharmacodynamic population parameter estimates [population variability coefficient of variation (CV)] for a maximum analgesic effect (Emax) model, in which the greatest possible pain relief (VAS 0–10) equates to an Emax of 10, were Emax 5.17 (64%) and 50% effective concentration 9.98 mg/l (107%). The equilibration half-life (teq) of the analgesic effect compartment was 53 min (217%). A placebo drug model for the effects of placebo response had a teq of 1.96 h (40%), an elimination half-life of 2.06 h (50%) and a potency of 1.54 pain relief units (24%). Conclusions: High dose acetaminophen (100 mg/kg) was no more effective than 40 mg/kg and was associated with increased nausea and vomiting. A target effect compartment concentration of 10 mg/l is expected to produce a pain reduction of 2.6 units. The placebo model accounted for a maximum pain reduction of 5.6 units at 3 h. The combination of placebo effect and preoperative acetaminophen 40 mg/kg results in pain scores below 4 units for 5 h postoperatively.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Accepted in revised form: 25 July 2001
Electronic Publication
Rights and permissions
About this article
Cite this article
Anderson, B.J., Woollard, G.A. & Holford, N.H. Acetaminophen analgesia in children: placebo effect and pain resolution after tonsillectomy. Eur J Clin Pharmacol 57, 559–569 (2001). https://doi.org/10.1007/s002280100367
Received:
Issue Date:
DOI: https://doi.org/10.1007/s002280100367