Abstract
Background
Noninvasive high frequency oscillatory ventilation through nasal prongs (nHFOV) has been proposed as a new respiratory support in neonatology. We studied the effect of ventilation parameters and nasal prongs on nHFOV efficacy and mechanics.
Methods
Customized sealed circuits connecting a SM3100A oscillator to a neonatal lung model were developed to evaluate the effect of applying HFOV via two different sized nasal prongs on delivered tidal volume and pressure. Measurements were made across a range of frequencies and pressures; amplitude was set to obtain visible lung oscillation.
Results
Volume delivered by peak-to-peak oscillation, ventilation, and pressure significantly differed among the interfaces, being higher for large cannulae and the control circuit (p < 0.0001). The interposition of a large or small nasal prong reduced volume to 56 and 26%, ventilation to 32 and 9%, and mean pressure to 83 and 79%, respectively, of the values measured for the direct connection of the oscillator to the test lung. Volume and ventilation were inversely related to frequency, which was particularly evident with larger diameter circuits due to higher delivered tidal volume (R 2 > 0.9). Increasing ventilation was associated with larger tidal volume and nasal prong diameter (adjusted R 2 = 0.97).
Conclusions
nHFOV using common nasal prongs is technically possible. Efficiency of tidal volume delivery is significantly affected by prong diameter.
Similar content being viewed by others
Introduction
In recent decades, high frequency oscillatory ventilation (HFOV) has received increasing attention in neonatal critical care [1] due to its theoretical lung protective characteristics. HFOV is still debated [2] but could be useful [3] to improve long-term respiratory outcome and reduce chronic lung disease in preterm infants [4, 5].
Continuous positive airway pressure through nasal cannulae (nCPAP) is usually provided to preterm neonates with respiratory failure for stabilizing proximal airways and surfactant-deficient alveoli, improving oxygenation, and reducing the need for intubation. Infants who fail nCPAP retain CO2 and may theoretically benefit from a noninvasive rescue ventilation; in these cases nasal conventional ventilation is widely used [6]. Due to its efficacy in eliminating CO2, HFOV using a nasopharyngeal tube has recently been proposed as a new respiratory support for preterm infants [7]. This should combine the properties of HFOV with the advantages of the noninvasive interface.
While many basic studies about endotracheal HFOV mechanics are available, no research has been conducted about HFOV efficacy using nasal cannulae (nHFOV) and about the mechanical effects of such an interface. Furthermore, relationships between parameter settings have not been evaluated under these conditions.
We built a bench model to study nHFOV efficacy and mechanics with different nasal prongs, with the secondary goal of analyzing the effect of various changes of frequency and mean airway pressure.
Materials and methods
Experimental circuits
A Sensormedics 3100A (Viasys Cardinalhealth, Dublin, OH, USA) was checked by the manufacturer before the study and then used for the experiments. The ventilator provides HFOV by transmitting the oscillation of a piston through a low-compliance tube specifically designed for this device. It displays the mean airway pressure (P aw), frequency, and peak-to-peak pressure amplitude (ΔP) set by the investigator.
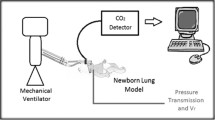
First, a circuit without nasal prongs was used as control. In this circuit the oscillator tubings were connected to a low-dead-space flow sensor. This was a hot-wire anemometer specifically designed for neonatal ventilators and connected to a respiratory function monitor (FLORIAN©, Acutronic Medical System, Zurich, Switzerland). This system has been proven to be reliable for measuring volumes at frequencies between 8 and 15 Hz [8]. To close the circuit, the flow sensor was then connected to a newborn lung model, routinely used for testing neonatal ventilators. This lung model (model 8409742, Draeger Medical, Lubeck, Germany) was composed of a “trachea” with an inner diameter 2.5 Fr (0.83 mm) and length 5 cm, and a test lung with a volume of 45 mL, compliance of 0.7 mL/cm H2O and resistance of 350 cm H2O L−1 s−1. In this circuit, tubings were connected in the same manner that occurs when testing the ventilator before applying it on an intubated patient.
We then built two experimental circuits in which the ventilator tubes were connected, through a shortened catheter mount, with nasal cannulae (Argyle CPAP nasal prongs; Sherwood Medical, St.Louis, MO, USA) of two different diameters, large (L) and small (S). These prongs have an outer diameter of 4.0 and 4.8 mm, respectively; inner diameter at both prong tips is 2.3 mm. Nasal cannulae were then connected and totally sealed to the flow sensor. The various parts of the circuit were sealed with cyanoacrylate, which was applied using a syringe and a 24 gauge angiocatheter to obtain the adhesive maximum control. Connections were then secured with silicon gel applied through a 14 gauge needle, and its crystallization was checked after 15 min.
The FLORIAN monitor was used to measure mechanical parameters distal to the nasal prongs. Volume generated by the peak-to-peak oscillation amplitude was considered as tidal volume (T v). Moreover, ventilation (DCO2) and mean airway pressure at the test lung (P L) were measured. DCO2 (calculated as frequency × T 2v ) represents the ventilation and provides a theoretical estimate of gas exchange during HFOV, therefore with tidal volumes smaller than the dead space [9, 10]. P L was measured using a line consisting of a low-compliance tube sealed to the bottom of the test lung and connected to the internal pressure transducer of the FLORIAN© monitor. We also calculated the difference P aw−P L for each measurement. The leak in the circuit was also registered; circuits were considered as closed and sealed if the leak was always <5%. The anemometer has a dead space of 1 mL and an accuracy of ±8%. The experimental setting is depicted in Fig. 1.
Experimental set-up
Once a circuit was completed, the oscillator was started and P aw was subsequently set to 10, 12, 16, and 20 cm H2O. For each P aw, the frequency was set at 7, 10, and 15 Hz, and the system was tested to be leak-free at all combinations of frequency and P aw. ΔP was set at the minimum value to obtain visible oscillation of the lung model and remained fixed at 45 cm H2O. Inspiratory time was set at 33%, and inspired oxygen fraction was at 0.40 for the entire study.
After this control phase, experiments were started for each combination of parameters. The experiments lasted 2 h for every circuit and data were acquired in real time every minute. A total of 120 measurements for every experimental setting were downloaded. Considering the measurement obtained in the control circuit to be 100%, the percentages of T v, DCO2, and P L for both experimental circuits were determined. Pressure drop [(P aw−P L)/P aw] for the two experimental circuits was also calculated in percent.
Statistics
Data were summarized as mean ± standard deviation and analyzed with ANOVA, and the Sidak test was used as post-hoc correction. Paired Student t test was utilized for comparing the percentages of nHFOV efficacy between the two types of nasal prongs.
Multiple curve estimation procedures were performed in order to find the best-fitting model to describe the relationship between ventilation parameters and measured variables. R 2 values were considered to assess the model goodness of fit, and linear or polynomial (second and third order) regressions were chosen since they presented the highest R 2 values [11, 12].
Multivariate linear analysis with backward stepwise method was also applied to the whole dataset to study the factors potentially influencing ventilation. Covariates inserted in the model were frequency and T 2v , which have a defined linear relationship with ventilation. We also included the type of interface used (small, large, or no prongs), since this presented a high linear relationship with the ventilation (Pearson coefficient r = 0.84; p < 0.001; adjusted R 2 of the linear model = 0.70). Condition index of eigenvalues was considered to estimate potential multicollinearity. R 2 values were considered to assess the multivariate model goodness of fit. Data were analyzed using SPSS for Windows ver. 15.0 (SPSS, Chicago, IL, USA), and p values <0.05 were considered to be significant.
Results
Table 1 presents basic mechanical data measured during the experiments. T v, DCO2, P L, and the difference P aw−P L showed overall significant differences among the three experimental settings. Post-hoc analysis revealed significant differences among circuits for all measurements: volume, ventilation, and distal pressure were higher for the circuit without nasal prongs and intermediate for the large cannulae. Consistently, the average difference P aw−P L was higher for small prongs and intermediate for large cannulae.
Figure 2 shows the relative efficacy of nHFOV over the range of frequencies or P aw set during the experiments. The interposition of a large or small nasal prong reduced tidal volumes to 56.3 ± 7.5 and 26.1 ± 4.8% (Fig. 2a), ventilation to 32.1 ± 6 and 9.2 ± 5.9% (Fig. 2b), and lung pressure to 83.2 ± 8.5 and 78.9 ± 9.4% (Fig. 2c), respectively, of the values for a direct connection of the oscillator circuit to the test lung (considered as 100%).
Efficiency of nHFOV. Delivered T v (a), DCO2 (b), and P L (c), for the different frequencies or P aw set during the experiments. Data are expressed as percentage of the same parameter measured in the control circuit (without nasal prongs). Solid horizontal lines represent 100% assumed for the control circuit. Black and gray/dashed lines interpolate all data measured during the entire experiment and represent the circuit with small and large prongs, respectively
The pressure drop was calculated as 38.5 ± 10.9% for small prong, 35.3 ± 10.1% for the large prong, and 22.1 ± 10.4% for the control circuit.
Figure 3 shows the relationship among mechanical parameters. T v was inversely correlated with the frequency (Fig. 3a). This was particularly evident with the large nasal prong and in the control circuit (R 2 > 0.9), whereas it was less significant when using smaller cannulae. The pressure difference (P aw−P L) increased as long as frequency increased, following a quadratic relationship (Fig. 3b). The pressure difference had a cubic relationship with T v and increased with decreasing T v. Moreover, at any volume, the difference increased with decreasing interface diameter, being highest with the smallest nasal cannula (Fig. 3c), which did not allow T v > 1 mL. No relationships were found between P aw and T v or P L and T v (R 2 ≈ 0).
Relationships among pressure decrease, T v, and frequency. Triangles represent control circuit data, while data obtained from circuits with large and small prongs are represented by circles and multiplication symbols, respectively. Black lines are the fitted data curve obtained with regression analyses. Line equations and R 2 values are shown
Table 2 presents the multivariate analysis results. A higher ventilation was primarily associated with higher T 2v , while the second most important factor was the type of interface used. Ventilation increased with the large cannula and even more with the circuit without nasal prongs. The model optimally fits data (adjusted R 2 = 0.97), and no relationship was found between covariates (eigenvalues condition index <10).
Discussion
To our knowledge, only two papers have been published about the use of nHFOV. A cross-over trial [7] conducted on 14 neonates recently confirmed the efficacy of nHFOV in eliminating CO2. Ten years before, a single case series hypothesized this efficacy, describing 21 neonates who were successfully ventilated using nHFOV [13]. These studies used a neonatal ventilator able to provide both conventional and high frequency ventilation; therefore babies were simply switched from CPAP to nHFOV, using just one preset frequency and leaving unchanged P aw/CPAP level. Although these reports are intriguing, there was neither appreciation about the potential benefits of the oscillatory waveform for maintaining airway patency, nor data about lung mechanics. Thus, available studies should be considered just as pilot experiences [14].
HFOV efficiency is significantly affected by the upper airways due to the motion of the pharyngeal wall and its variable compliance [15, 16]. Several other factors are also important [17]: the tube diameter, the frequency used, the mechanical characteristics of the ventilated lung, and technical properties of the machine are only some of these factors. Furthermore, the efficiency of nHFOV could also be affected by the interface. In fact, different nasal prongs are known to carry variable resistances [18]. Since exploring all variables in a single study would not be possible, we chose to build an experimental model focused on the following factors: (1) the interface, studying two different types of nasal prongs, which are the most common device to provide noninvasive ventilation to neonates; and (2) the change in frequency, keeping ΔP unchanged to be more able to distinguish differences. Moreover, we decided to use the Sensormedics 3100A device, the most powerful available piston oscillator, because of its well known ability to provide an active expiration phase and to generate adequate tidal volumes at a wide range of frequencies [19, 20]. Finally, in order to simulate the real clinical context, a lung model carrying the mechanical properties of the recovery phase of hyaline membrane disease was coupled with the oscillator. This commercially available test lung was a simple and pragmatic choice for mimicking the clinical scenario.
We studied the mechanical properties of HFOV with two noninvasive interfaces, and we found significant differences in T v and DCO2. This is consistent with the relationship between endotracheal tube diameter and T v and DCO2, already described for HFOV [19, 20]. Smaller cannulae lower T v reaching the distal airways, and this phenomenon was much more evident with the smaller nasal prongs, which delivered ≈26% of the T v measured in the control circuit. Figure 2 also shows that the theoretical CO2 elimination provided by small cannulae would be only ≈9% of that measured in the reference circuit. The magnitude of the pressure drop is also affected by the interface diameter. In fact, the drop along the circuit increased as the diameter of the cannulae decreased. This is consistent with available data on animals under endotracheal HFOV, which show a high pressure drop between the set P aw and the tracheal and the alveolar pressure. In detail, a 97% pressure drop along the trachea and a 75% drop between trachea and alveoli have been demonstrated in rabbits [21]. We did not use an animal model, which would be useful but very difficult to create for studying nasal ventilation, therefore we cannot offer any thoughts about the “real” alveolar pressure. Nevertheless, our bench model may account for the tracheal pressure drop, since our “lung” includes an artificial trachea of suitable characteristics.
Regression analyses also show data (Fig. 3; Table 2) consistent with those measured in endotracheal HFOV studies (although we must highlight that, in our findings, DCO2 is only a theoretical estimate of the gas exchange) [22–24]. Furthermore, frequency determines the time allowed for the piston displacement and so the volume supplied, such that lower frequencies allow greater T v [22] and there is an augmented resistance to flow with increasing tidal volumes. During nHFOV, this phenomenon is less evident with the smallest nasal prongs: the T v reduction caused by this interface is probably enough to mask the effect of frequency on T v. Consequently, using smaller prongs, DCO2 did not significantly increase with decreasing frequencies and was too small to guarantee a significant ventilation (data not shown). These considerations need to be verified looking at lower frequencies, seldom used in neonates, to clarify if there is a steep rise in T v under such conditions. In that case the lack of effect of frequency on T v might be due to the relatively high compliance of the prongs. Since an ideal T v in HFOV should be between 1 and 2 mL/kg, based on our data, large prongs could be better but must be weighted against the “size” of the infant’s nares.
Consistently, data about pressure differences (P aw−P L) were also in line with previous findings from Pillow and coworkers [25]. This difference increases in a polynomial curve with increasing frequency or decreasing T v. At any T v, the pressure drop increases with the decreasing interface diameter. Therefore, reduction in the model resistance, by increasing interface diameter, diminished the pressure drop, which was highest with the smallest nasal cannula and lowest in the control circuit. Moreover, at lower T v, P L fell more quickly than at higher volumes.
Finally, the lack of relationship between P aw or P L and T v is also in agreement with earlier descriptions of the performances of the oscillator [20], which is able to generate subatmospheric expiratory pressures at the inferior peak of the ΔP to guarantee an adequate volume. Multivariate analysis confirmed this data interpretation: T 2v and the type of interface are the main factors associated with DCO2, which increases with increasing T v and larger prongs.
Model limitations
Our experimental model cannot account for all phenomena occurring in vivo, and some study limitations make our findings preliminary to further preclinical studies. First, the study of a few frequencies might have hidden a nonlinear relationship between T v and the frequency. We analyzed frequencies in the common neonatal range, but more than three frequency values should be studied to verify this relationship and to allow for an appropriate T v to be found with lower frequencies.
Second, volumes measured under the present experimental conditions could be significantly greater than in clinical practice. In fact, pressure oscillations are likely to be further dampened by the increased dead space of the pharynx [7]. Conversely, potential benefits of the oscillatory waveform on airway patency should also be verified, since some evidence seems to indicate that direct lung ventilation is less important [9] and gas exchange during HFOV might be improved by the flow fluctuation in the pharyngolaryngeal area [10, 13].
Third, the in vivo circuit should be regarded as an “open” circuit. In fact, our model works because of the validity of the seal; this is not likely to be duplicated in human neonates with leaks occurring through the mouth or if too small prongs are used. Moreover, secretions and esophageal pressure variations may affect the nHFOV performance, and active exhalation could be injurious, exerting a significant pressure on the nasal mucosa. Special nostril care could be needed to prevent skin damages, especially when nHFOV is applied over the long-term. Finally, since different compliances and resistances are known to affect the oscillator performance [20], our considerations only apply to preterm lungs affected by hyaline membrane disease, as represented by our model. nHFOV has been provided to neonates with various lung injury types [7, 13]. It is important to note that lower compliance may result in increasing oscillation and P L transmission, while higher airway resistances (i.e., bronchopulmonary dysplasia) may decrease them [22].
Conclusions
We provided preliminary insights into the mechanics of nHFOV, determining that it is technically possible, when comparing it to endotracheal HFOV, notwithstanding that a significant pressure drop occurs. Nasal prong diameter and flow are the main determinants of the delivered T v and DCO2. Larger prongs might provide a better ventilation but the choice of adequate interface must consider the size of the infant’s nostrils, and thus potential leaks and the risk of skin damages. Before embarking on a clinical trial, a specific bench study should investigate the effects of different amplitudes. In vivo measurements of oscillation transmission, using different interfaces, should also be undertaken.
Abbreviations
- CO2 :
-
Carbon dioxide
- DCO2 :
-
Ventilation during HFOV
- HFOV:
-
High frequency oscillatory ventilation
- T v :
-
Tidal volume
- nHFOV:
-
Nasal noninvasive high frequency oscillatory ventilation
- nCPAP:
-
Nasal continuous positive airway pressure
- P aw :
-
Mean pressure at the airway opening
- ΔP :
-
Peak-to-peak pressure amplitude
- P L :
-
Distal pressure measured at the lung model
References
Greenough A, Premkumar M, Patel D (2008) Ventilatory strategies for the extremely premature infant. Pediatr Anesth 18:371–377
Henderson-Smart DJ, De Paoli AG, Clark RH, Bhuta T (2009) High frequency oscillatory ventilation versus conventional ventilation for infants with severe pulmonary dysfunction born at or near term. Cochrane Database Syst Rev 3:CD002974
Zecca E, De Luca D, Costa S, Marras M, Romagnoli C (2006) Neonatal intensive care and outcomes of extremely preterm infants: changes over a decade. Ital J Pediatr 32:48–54. http://www.ijponline.net
Henderson-Smart DJ, Cools F, Bhuta T, Offringa M (2007) Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst Rev 3:CD000104
Hofhuis W, Huysman MW, van der Wiel EC, Holland WP, Hop WC, Brinkhorst G, de Jongste JC, Merkus P (2002) Worsening of V′max FRC in infants with chronic lung disease in the first year of life: a more favorable outcome after high frequency oscillation ventilation. Am J Resp Crit Care Med 166:1539–1543
Owen LS, Morley CJ, Davis PG (2008) Neonatal intermittent positive pressure ventilation: a survey of practice in England. Arch Dis Child Fetal Neonatal Ed 93:F148–F150
Colaizy TT, Younis UMM, Bell EF, Klein JM (2008) Nasal high frequency ventilation for premature infants. Acta Paediatr 97:1518–1522
Scalfaro P, Pillow JJ, Sly PD, Cotting J (2001) Reliable tidal volume estimates at the airway opening with an infant monitor during high-frequency oscillatory ventilation. Crit Care Med 29:1925–1930
Chang HK (1984) Mechanisms of gas transport during ventilation by high frequency oscillation. J Appl Physiol 56:553–564
Boynton BR, Hammond MD, Fredberg JJ, Buckley BG, Villanueva D, Frantz ID (1989) Gas exchange in healthy rabbits during high frequency oscillatory ventilation. J Appl Physiol 66:1343–1351
Norusis M (2004) SPSS 13.0 advanced statistical procedures companion. Prentice Hall, Upper Saddle-River
Cameron AC, Windmeijer FAG (1997) An R-squared measure of goodness of fit for some common nonlinear regression models. J Econometrics 77:329–342
van der Hoeven M, Brouwer E, Blannco CE (1998) Nasal high frequency ventilation in neonates with moderate respiratory insufficiency. Arch Dis Child Fetal Neonatal Ed 79:F61–F63
Carlo WA (2008) Should nasal high frequency ventilation be used in preterm infants? Acta Paediatr 97:1484–1485
Desager KN, Cauberghs M, Naudts J, van de Woestijne KP (1999) Influence of upper airway shunt on total respiratory impedance in infants. J Appl Physiol 87:902–909
Loos N, Peslin R, Marchal F (2000) Respiratory and upper airways impedance responses to methacholine inhalation in spontaneously breathing cats. Eur Resp J 15:1001–1008
Fredberg JJ, Glass GM, Boynton BR, Frantz ID (1987) Factors influencing mechanical performance of neonatal high frequency ventilators. J Appl Physiol 62:2485–2490
De Paoli AG, Morley CJ, Davis PG, Lau R, Hingeley E (2002) In vitro comparison of nasal continuous positive airway pressure devices for neonates. Arch Dis Child Fetal Neonatal Ed 86:F42–F45
Hatcher D, Watanabe H, Ashbury T, Incent S, Fisher J, Froese A (1998) Mechanical performance of clinically available, neonatal, high-frequency, oscillatory-type ventilators. Crit Care Med 26:1081–1088
Jouvet P, Hubert P, Isabey D, Pinquier D, Dahan E, Cloup M, Harf A (1997) Assessment of high frequency neonatal ventilator performances. Intensive Care Med 23:208–213
Gerstmann DR, Fouke JM, Winter DC, Taylor AF, de Lemos RA (1990) Proximal, tracheal and alveolar pressures during high frequency oscillatory ventilation in a normal rabbit model. Pediatr Res 28:367–373
Derdak S (2003) High frequency oscillatory ventilation for acute respiratory distress syndrome in adult patients. Crit Care Med 31(Suppl):S317–S323
Schindler M, Seear M (1991) The effect of lung mechanics on gas transport during high frequency oscillation. Pediatr Pulmonol 11:335–339
Boynton BR, Carlo WA (1994) Pulmonary gas exchange during high frequency ventilation. In: Boyton BR, Carlo WA, Jobe AH (eds) New therapies for neonatal respiratory failure. A physiological approach. Cambridge University Press, Cambridge, pp 218–244
Pillow JJ, Sly PD, Hantos Z, Bates JHT (2002) Dependency of intrapulmonary pressure amplitude on respiratory mechanics during high frequency oscillatory ventilation in preterm lambs. Pediatr Res 52:538–544
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Study results were presented in part as oral communication to the 50th Annual Meeting of the European Society for Pediatric Research (ESPR) held in Hamburg (Germany), 10 October 2009.
Rights and permissions
About this article
Cite this article
De Luca, D., Carnielli, V.P., Conti, G. et al. Noninvasive high frequency oscillatory ventilation through nasal prongs: bench evaluation of efficacy and mechanics. Intensive Care Med 36, 2094–2100 (2010). https://doi.org/10.1007/s00134-010-2054-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-2054-7