Abstract
Objective
In addition to the previous classification of chronic lung disease (CLD) O2 dependency at 36 weeks of postmenstrual age, a new definition of CLD has recently been proposed: new bronchopulmonary-dysplasia (BPD). This uses total duration of O2 supplementation and positive pressure requirements to delineate three degrees of severity (mild, moderate, and severe) according to the respiratory status at 36 weeks postmenstrual age. We analyzed the balance of serum proinflammatory and profibrotic/angiogenic cytokine concentrations in relation to CLD and the new BPD definition.
Design and setting
Descriptive study in a third-level neonatal ICU.
Patients
Thirty-one preterm neonates with a gestational age of 24–29 weeks were studied to evaluate their serum cytokine concentration; they were previously enrolled in a randomized clinical trial to compare the effects of high-frequency oscillatory ventilation vs. intermittent mandatory ventilation in terms of pulmonary mechanics and lung cytokines. Serum samples were collected on days 1, 3, and 5 after birth until extubation to detect the levels of three proinflammatory cytokines plus four profibrotic/angiogenic cytokines, and correlations were examined to old CLD and new BPD. Ventilation treatments were distributed homogeneously between the groups and did not interfere with the results presented here.
Results and conclusions
Old CLD development, mainly corresponding to the moderate/severe forms of new BPD, was associated with increased proinflammatory and profibrotic/angiogenic cytokines, while mild forms of new BPD were characterized only by increases in profibrotic/angiogenic cytokines, suggesting a different balance of two pathogenic mechanisms in different phases of the disease.
Similar content being viewed by others
Introduction
Our group has recently published data regarding cytokine levels in the epithelial lining fluid (ELF) of preterm newborns as a function of the ventilation strategies of high-frequency oscillatory ventilation (HFOV) and synchronized intermittent mandatory ventilation (sIMV) adopted during the first week of life [1]. The report showed that transforming growth factor (TGF) β1 ELF levels were early and significantly lower in the HFOV group than in sIMV-treated babies. In addition, HFOV was associated with significantly less chronic lung disease (CLD). In most of these subjects we also determined the serum levels of the cytokines and found in HFOV-treated newborns lower concentrations of the proinflammatory interleukin (IL) 6 than in the sIMV group [2]. In contrast to a large body of data regarding cytokine concentrations in the bronchoalveolar lavage fluid (BALF) of premature newborns in relation to CLD development [1, 3, 4, 5, 6, 7, 8] or ventilatory treatments or infection [9], very little is known regarding their serum levels as a function of their classification for CLD [10].
The most widely used definition of CLD in premature newborns is currently that of O2 dependency at 36 weeks postmenstrual age, suggested as the best predictor of long-term respiratory outcomes [11]. The children affected by CLD present over time with various symptoms such as airflow limitation, gas trapping, exercise intolerance, and pulmonary hypertension. A revision of the BPD definition for infants with gestational age under 32 weeks has recently been proposed by Jobe and Bancalari [12] (“new BPD”). This uses total duration of O2 supplementation (at least 28 days with oxygen > 21% concentration) and positive pressure requirements to differentiate three degrees of BPD severity depending on respiratory status at 36 weeks postmenstrual age or at discharge, whichever comes first: mild (breathing room air), moderate (breathing oxygen < 30%), and severe (requiring ≥ 30% oxygen and/or positive pressure).
New BPD is characterized more by arrested lung development and impaired alveolar and vascular growth than inflammatory processes [13]. On the basis of the histological picture of the lung, some authors [13, 14, 15] have noted that conducting airways and gas exchanging area developments are interdependent processes. In addition, in the presurfactant era airway injury, inflammation, and parenchymal fibrosis were the prominent findings in BPD. Thereafter lung histology showed a more uniform inflation, with fewer and larger alveoli, indicating an interference with septation. Moreover, decreased microvascular development was observed in some patients. Surfactant and oxygen concentration may strongly influence the histology and clinical picture also in function of prematurity, at least in animal models [16, 17]. The statement that old CLD is no longer seen today is not completely true, as lung radiograms shows that fibrosis and inflammation are still present even in the postsurfactant era [4].
We examined newborns previously enrolled in our randomized clinical trial [1] to evaluated cytokine serum levels in relation to old CLD and new BPD [12]. Our hypothesis was that a different balance of inflammatory and profibrotic/angiogenic cytokines characterize these conditions. The cytokines measured in our study are representative of the proinflammatory group (IL-6, IL-8, and monocyte chemotactic peptide 1, MCP-1) or the anti-inflammatory group (IL-10, TGF-β1). In addition, vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF) BB, and TGF-β1 are also representative cytokines of the profibrotic/angiogenic group of mediators, which provide information about possible associations between the pathological outcomes in premature babies and very early modifications of blood cytokine concentrations.
Materials and methods
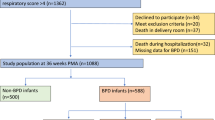
This study was carried out in our third-level neonatal intensive care unit (NICU) between July 2000 and January 2003. Several procedural characteristics have already been published and may be found in detail in our previous report [1]. Briefly, neonates with a birth weight of 500–1500 g and gestational age of 24–29 weeks were eligible when inborn, endotracheal intubation was required at birth, and on-going intensive care was required. They were randomly assigned to HFOV or sIMV within 30 min from birth. Babies with major congenital malformations or prenatal infection were excluded from the study. The study protocol and consent forms were approved by the Ethics Committee of the Department of Pediatrics.
During the 30-month study period 479 newborns were admitted to our NICU, including 445 inborns (92.9%), and 34 outborns (7.1%). Seventy of these had a gestational age of 24–29 weeks (i.e., at highest risk of CLD), and 42 met the entry criteria. Two neonates (one in each group) with late-diagnosed congenital pneumonia (positive BALF culture at birth) were subsequently excluded. Six patients were extubated within the first 24 h of life, prior to serum sample collection. Additionally, three patients died before 36 weeks of age (one in the HFOV group, two in the sIMV group) and were excluded from final analysis. This study thus followed 15 patients receiving HFOV and 16 receiving sIMV. No infant needed to be ventilated again within the next 72 h following extubation. Two infants in sIMV group died with the endotracheal tube in place.
Ventilation strategies
The goals of respiratory management are to maintain blood gas at pH 7.30–7.45, PaCO2 45–55 mmHg (5.9–7.2 kPa) and PaO2 50–70 mmHg (6.6–9.3 kPa) with oxygen saturation 90–94%. HFOV and sIMV were performed with the Draeger Babylog 8000 plus (Draeger, Lübeck, Germany), as previously described [1]. Extubation was attempted when the neonate's condition remained stable for at least 6 h while receiving minimal ventilation. In the HFOV group this was at: FIO2 0.25 or less, MAP less than 6 cmH2O and an amplitude below 30%; in the sIMV group it was at: peak inspiratory pressure 18 cmH2O or less, ventilator rate 15 breaths per minute, and FIO2 0.25 or less. Babies in both groups were extubated on nasal continuous positive airway pressure of 4–5 cmH2O (nasal prongs Argyle, Sherwood Medical, St. Louis, Mo., USA). Extubation failure was defined in both groups as no longer than 72 h, with clinical deterioration requiring reintubation.
Medical treatment
Surfactant (a pig-derived, natural surfactant, Curosurf, Chiesi Farmaceutici, Parma, Italy) was administered at a dose of 200 mg/kg, and a second dose of 100 mg/kg was used in babies receiving sIMV if the inspired oxygen concentration was greater than 30%, and in those receiving HFOV if MAP was greater than 10 cmH2O. All studied neonates were given intravenous ibuprofen as lysine salt (Arfen, Lysafarma, Erba-Como, Italy) as prophylaxis for patent ductus arteriosus according to our previous experience. All babies were initially treated with antibiotics (ampicillin and amikacyn) for the first 7 days of life to prevent lung colonization or infection. Caffeine citrate (caffeine citrate 10 mg/ml; Monico, Venice, Italy) was administered intravenously at a daily dose of 5 mg/kg after a loading dose of 20 mg/kg from birth to the 33rd week of postconceptual age to prevent apneic spells.
Cytokine determinations
After initial stabilization and after surfactant therapy had been administered, serum samples were obtained at the end of the first day of life and on postnatal days 3 and 5 unless there was early extubation. Commercially available enzyme linked immunosorbent assay kits (IL-6, IL-8, IL-10, MCP-1, PDGF-BB, VEGF, TGF-β1 R&D Systems Europe, Abingdon, UK) were used following manufacturer's instructions.
Statistical analysis
Categorical variables were compared using the two-tailed Fisher's exact test. Both parametric and nonparametric tests were used as necessary. The statistical software used included Instat (GraphPad PRISM version 3.02) and Epi-Info 2000. Differences with a p value less than 0.05 were considered statistically significant. A power analysis, based on serum cytokine levels could not be performed due to the lack of sufficient data.
Results
Babies were subdivided into groups according to the definitions of CLD and new BPD. Several variables were analyzed, but only four differed significantly between CLD groups and only one between new BPD groups (Table 1). CLD-positive and CLD-negative groups differed for two variables linked to the babies—birth weight and intrauterine growth retardation—and two linked to outcome—extubation time and survival. New BPD groups differed significantly only in birth weight. These differences are in part due to the relationship between the two classifications (Table 2). It was the case that “no and mild new BPD” patients were included in the CLD-negative group while “moderate and severe new BPD” patients were included in the CLD-positive group. Ventilation strategies were randomly assigned and balanced between the groups so that interferences due to ventilation type were eliminated. The limited number of patients did not permit a double stratification of patients (ventilation and outcome).
Table 3 reports the variations in serum cytokine levels in CLD-positive and CLD-negative groups. On day 1 no differences were observed, while on days 3 or 5 only pro-inflammatory cytokines (IL-6, IL-8, MCP-1) were significantly lower in CLD-negative than in CLD-positive newborns, plus IL-10, which is known to often behave similarly, possibly to block the inflammatory mechanisms. In addition, the concentrations of IL-6, IL-8, and IL-10 declined significantly over time only in CLD-negative subjects. No significant differences were found in the levels of the profibrotic/angiogenic cytokines TGF-β1, PDGF-BB, and VEGF.
Table 4 shows the variations in same cytokine levels in the groups defined by the new BPD classification. Here we observed significantly higher levels of profibrotic cytokines (TGF-β1, PDGF-BB, VEGF) on day 5 in the new BPD-positive group. In addition, in the new BPD-negative group, one defined similarly to the CLD-negative group, a significant reduction in IL-6 was also confirmed, although with a limited significance level.
Due to the overlapping features of the CLD and new BPD classifications, as shown in Table 2, the variables reported in Tables 1, 3, and 4 differentiate three groups of patients: CLD-negative, new BPD-negative (group A), CLD-negative, new BPD-positive (mild forms; group B), and CLD-positive, new BPD-positive (moderate and severe forms; group C). Table 5 reports some characteristics of the three groups. As expected, birth weight was significantly lower and extubation time significantly longer in group C than in groups A and B. In addition, on day 1 inflammatory MCP-1 and IL-8 cytokines exhibited sequentially increased values, shifting from group A to group C (p < 0.05). None of the other variables showed significant differences between the three groups.
As shown in Table 4, changes in the pro-fibrotic/angiogenic factors were evident only on day 5, where the number of patients was drastically reduced by the extubation procedure. Therefore Table 5 also reports the behavior of TGF-β1 on day 5. TGF-β1 levels increased in groups B and C, which included the new BPD-positive patients, although the data did not differ significantly for the reduced number of patients. The other two fibrotic/angiogenic cytokines (PDGF and VEGF) behaved similarly.
Discussion
Our findings show that subjects classified by the “old” CLD definition initially (day 1) did not differ in serum cytokine levels. However, during the subsequent 5 days serum proinflammatory IL-6 and IL-8 concentrations (day 3 and 5), MCP-1 (day 5), and anti-inflammatory IL-10 (day 3) increased significantly in CLD-positive infants (Table 3). In several models (pemphigo, psoriasis), serum IL-10 followed the behavior of proinflammatory cytokines, possibly to block proinflammatory cytokines effects [18, 19].
Interestingly, when patients were divided in terms of new BPD, we observed a shift of significance from proinflammatory cytokines to the other cytokines, with all three angiogenic/profibrotic factors (TGF-β1, PDGF-BB, VEGF) significantly increased on day 5 in new BPD-positive infants. These data suggest that this classification redistributes subjects, as serum levels of TGF-β1, PDGF-BB, and VEGF are altered on day 5 (Table 4).
We combined mild, moderate, and severe forms of new BPD vs. patients without new BPD for two reasons: (a) we lacked enough patients to allow a reasonable number in each subgroup, and (b) no differences in the incidence of comorbidities associated with different grades of newly defined BPD were found in our population. Moreover, recent data by Sahni et al. [20] show no differences between mild, moderate, and severe forms, except for patent ductus arteriosus requiring surgical ligation (ibuprofen prophylaxis was given in our patients).
To better compare the two classifications we differentiated patients into three groups: CLD-negative, new BPD-negative (group A), CLD-negative, new BPD-positive (mild form; group B), and CLD-positive, new BPD-positive (moderate and severe forms; group C). MCP-1 and IL-8 levels differed significantly between the three groups and increased sequentially from group A to the group C, suggesting that in the “old type” of CLD lung inflammation mechanisms predominate very early (day 1). At the same time, no differences were observed in the profibrotic modulators. Profibrotic mediators tended to change later. TGF-β1 levels showed increases in groups B and C only on day 5, albeit without statistical significance. It is important to remember that due to infant extubation the number of patients was smaller on day 5 than on day 1. These data support the hypothesis that the “new type” of BPD is characterized principally by modifications in profibrotic markers associated with impaired alveolar and vascular growth.
Cytokines are the fundamental regulators of cell interactions and drive their functions. In particular, some of these molecules belong to the group of inflammatory cytokines, such as IL-2, IFN-γ, IL-6, IL-8, MCP-1, tumor necrosis factor (TNF) α and many others. These substances induce the inflammatory processes, recruiting and stimulating the inflammatory cells. Other cytokines, sometimes termed anticytokines, exert anti-inflammatory, profibrotic actions, also regulating the neo-angiogenic processes. This group includes IL-4, IL-10, TGF-β1, VEGF, and PDGF-BB modulators [21, 22].
Although the new BPD definition was introduced in 2001, there is very little information regarding the cytokine levels in sera of newborns during the first days of life [2, 10]. In contrast, there are many studies concerning BALF levels of several cytokines [1, 3, 4, 5, 6, 7, 8, 9] of both T helper 1 and T helper 2 origin [22]. Notwithstanding some controversial findings, all of these conclude that premature newborns who develop BPD/CLD show significantly elevated proinflammatory cytokines levels (IL-1, IL-6, IL-8 and TNF-α being the most studied) [5, 6, 7] as well as MCP-1 (a CC chemokine particularly activating the lung macrophages) [8] and IL-10 [7]. In addition, IL-6, IL-8, and TNF-α were also increased in the cord blood as predictors of CLD [23]. Only one study has reported IL-6, IL-1β, and TNF-α levels in the blood of subjects classified as new BPD [24], but no association has been found between the fetal inflammatory response and BPD risk. Birth weight (and not gestational age) and extubation time are the only clinical factors differing significantly between subjects developing or not developing CLD (Table 5), according to recent data showing a significantly greater risk of developing CLD in SGA infants [25].
As previously shown, HFOV determines various cytokine levels in BALF and sera of premature infants when they are subjected to ventilation [1, 2]. In this study we could not obtain data stratified for different ventilation strategies plus outcome (CLD or new BPD) due to the limited number of patients. However, any interference by ventilation treatments could be eliminated through its homogeneous distribution, although a prevalence of sIMV-treated babies was observed in the CLD-positive group (Table 1) and in the severe forms of new BPD (Table 2). Cytokine production may vary in function of the prematurity degree or oxygen intake after birth [21]. Gestational age did not differ between our study groups, as reported in Table 1. However, birth weight did differ significantly due to the number of small for gestational age newborns included in the CLD and new BPD groups. Therefore we may presume that prematurity did not affect the comparisons. On the basis of clinical symptoms and laboratory tests, no cases of sepsis/infection was detected during the 5 days of newborn observation. Therefore we can assume that there was no interference by infections on the serum cytokine concentrations.
Limits of the present study include the relatively low number of the subjects analyzed. In particular, we report only 3 moderate forms of new BPD and 11 of mild BPD. This may be due to the enrollment rules of our previous study [1], causing us to lose some neonates not intubated, receiving only continuous positive airway pressure or O2 by nasal cannula and possibly developing CLD. During the period of this study only two newborns were not ventilated but received nasal continuous positive airway pressure and O2 therapy and developed CLD, categorized as mild new BPD. None of the other infants without mechanical ventilation developed CLD. Therefore the limited number of unincluded newborns cannot represent an important bias.
In conclusion, it is possible that with the new definition of BPD only the mild forms accord with the theory of impaired lung maturation suggested by increases in neo-angiogenic modulators, while the moderate/severe forms are characterized by both inflammatory and fibrotic processes. The results presented here are in agreement with two possible main mechanisms, the first being prevalently inflammatory (overt forms of CLD/BPD) and the second angiogenic (maturative with impaired alveolar and pulmonary vascular development coinciding with the mild forms of new BPD). An early screening of a few serum mediators may be useful for defining the risk of developing mild or severe forms of chronic lung disease of the newborns, addressing a potential need for a different selection of therapies, in particular corticosteroids and surfactant at varying doses.
References
Vento G, Matassa PG, Ameglio F, Capoluongo E, Zecca E, Tortorolo L, Martelli M, Romagnoli C (2005) HFOV in premature neonates: effects on pulmonary mechanics and epithelial lining fluid cytokines. A randomized controlled trial. Intensive Care Med 31:463–470
Capoluongo E, Vento G, Santonocito C, Matassa PG, Vaccarella C, Giardina B, Romagnoli C, Zuppi C, Ameglio F (2005) Serum levels of seven cytokines in premature newborns treated with two ventilatory procedures: HFOV and sIMV. Differences in IL-6, IL-8 and IL-10 serum values. Eur Cytokine Netw 16:199–205
Tullus K, Noack GW, Burman LG, Nilsson R, Wretlind B, Brauner A (1996) Elevated cytokine levels in tracheobronchial aspirate fluids from ventilator treated neonates with bronchopulmonary dysplasia. Eur J Pediatr 155:112–116
Vento G, Matassa PG, Ameglio F, Capoluongo E, Tortorolo L, Romagnoli C (2002) Effects of early dexamethasone therapy on pulmonary fibrogenic mediators and respiratory mechanics in preterm infants. Eur Cytokine Netw 13:207–214
Kazzi SN, Romero R, McLaughlin K, Ager J, Janisse J (2001) Serial changes in levels of IL-6 and IL-1beta in premature infants at risk for bronchopulmonary dysplasia. Pediatr Pulmonol 31:220–226
Munshi UK, Niu JO, Siddiq MM, Parton LA (1997) Elevation of interleukin-8 and interleukin-6 precedes the influx of neutrophils in tracheal aspirates from preterm infants who develop bronchopulmonary dysplasia. Pediatr Pulmonol 24:331–336
Beresdorf MW, Shaw NJ (2002) Detectable IL-8 and IL-10 in bronchoalveolar lavage fluid from preterm infants ventilated for respiratory distress syndrome. Pediatr Res 52:973–978
Baier RJ, Majid A, Parupia H, Loggins J, Kruger TE (2004) CC chemokine concentrations increase in respiratory distress syndrome and correlate with development of bronchopulmonary dysplasia. Pediatr Pulmonol 37:137–148
Andrews P, Azoulay E, Antonelli M, Brochard L, Brun-Buisson C, Dobb G, Fagon JY, Gerlach H, Groeneveld J, Mancebo J, Metnitz P, Nava S, Pugin J, Pinsky M, Radermacher P, Richard C, Tasker R, Vallet B (2005) Year in review in intensive care medicine, 2004. I. Respiratory failure, infection, and sepsis. Intensive Care Med 31:28–40
Gitto E, Reiter RJ, Amodio A, Romeo C, Cuzzocrea E, Sabatino G, Buonocore G, Cordaro V, Trimarchi G, Barberi I (2004) Early indicators of chronic lung disease in preterm infants with respiratory distress syndrome and their inhibition by melatonin. J Pineal Res 36:250–255
Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM (1988) Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 82:527–532
Jobe AH, Bancalari E (2001) Bronchopulmonary dysplasia. Am J Respir Crit Care Med 163:1723–1729
Burri PH (1997) Structural aspects of prenatal and postnatal development and growth of the lung. In: McDonald JA (eds) Lung growth and development. Dekker, New York, pp 1–35
Tschanz SA, Burri PH (1997) Postnatal lung development and its impairment by glucocorticoids. Pediatr Pulmonol Suppl 16:247–249
Hussain NA, Siddiqui NH, Stocker JR (1998) Pathology of arrested acinar development in post-surfactant bronchopulmonary dysplasia. Hum Pathol 29:710–717
Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA (1999) Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med 160:1333–1346
Albertine KH, Jones GP, Starcher BC, Bohnsack JF, Davis PL, Cho S, Carlton DP, Bland RD (1999) Chronic lung injury in preterm lambs. Am J Respir Crit Care Med 159:945–958
D'Auria L, Cordiali Fei P, Ameglio F (1999) Cytokines and bullous pemphigoid. Eur Cytokine Netw 10:123–134
Bonifati C, Ameglio F (1999) Cytokines in psoriasis. Int J Dermatol 38:241–251
Sahni R, Ammari A, Suri MS, Milisavljevic V, Ohira-Kist K, Wung JT, Polin RA (2005) Is the new definition of bronchopulmonary dysplasia more useful? J Perinatol 25:41–46
Jankov RP, Keith Tanswell A (2004) Growth factors, postnatal lung growth and bronchopulmonary dysplasia. Paediatr Respir Rev 5:S265
Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL (1986) Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 136:2348–2357
An H, Nishimati S, Ohyama M, Haruki A, Naruto T, Kobayashi N, Sugai T, Kobayashi Y, Mori M, Seki K, Yokota S (2004) Interleukin-6, interleukin-8, and soluble tumor necrosis factor receptor-I in the cord blood as predictors of chronic lung disease in premature infants. Am J Obstet Gynecol 191:1649–1654
Viscardi RM, Muhumuza CK, Rodriguez A, Fairchild KD, Sun C-CJ, Gross GW, Campbell AB, Wilson PD, Hester L, Hasday JD (2004) Inflammatory markers in intrauterine and fetal blood and cerebrospinal fluid compartments are associated with adverse pulmonary and neurologic outcomes in preterm infants. Pediatr Res 55:1009–1017
Lal MK, Manktelow BN, Draper ES, Field DJ (2003) Chronic lung disease of prematurity and intrauterine growth retardation, a population based study. Pediatrics 111:483–487
Acknowledgements
We thank Prof. E. Bancalari, Chief of the Division of Neonatology, University of Miami, Florida, USA, for suggestions in evaluating the results of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vento, G., Capoluongo, E., G. Matassa, P. et al. Serum levels of seven cytokines in premature ventilated newborns: correlations with old and new forms of bronchopulmonary dysplasia. Intensive Care Med 32, 723–730 (2006). https://doi.org/10.1007/s00134-006-0138-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-006-0138-1